Etiology
Aortic regurgitation (AR) is primarily caused by diseases of the aortic valve itself or the aortic root. It can be classified as acute AR and chronic AR based on the onset of the condition.
Acute Aortic Regurgitation
The main causes include:
- Infective endocarditis
- Chest trauma: Leading to rupture or prolapse of the aortic root, supporting structures, or valve leaflets
- Aortic dissection: Hematoma causing dilation of the aortic annulus or tears the valve leaflets or annulus
- Prosthetic valve rupture
Chronic Aortic Regurgitation
Diseases of the aortic valve include:
- Rheumatic heart disease
- Congenital abnormalities
- Infective endocarditis
- Degenerative aortic valve disease
- Aortic valve prolapse
Rheumatic Heart Disease
Approximately 2/3 of AR cases are caused by rheumatic heart disease, often associated with aortic stenosis and mitral valve disease.
Congenital Abnormalities
Bicuspid aortic valve accounts for 1/4 of isolated AR cases. In children, regurgitation is often due to cusp notching or prolapse of a large, elongated cusp into the left ventricle. In adults, it is more commonly caused by progressive cusp fibrosis, retraction, or secondary infective endocarditis.
Infective Endocarditis
It is a common cause of isolated AR, resulting from leaflet damage or perforation caused by vegetations. Prolapse may occur due to damage to supporting structures, or vegetations between leaflets may prevent proper closure, leading to regurgitation. Even after infection control, fibrosis and retraction of the leaflets can persist.
Degenerative Aortic Valve Disease
75% of older patients with degenerative calcific aortic stenosis also have AR.
Aortic Valve Prolapse
It is caused by myxomatous degeneration of the valve, leading to diastolic prolapse of the cusps into the left ventricle, occasionally associated with cystic medial necrosis of the aortic root, which may have a congenital origin.
Aortic annular dilation prevents proper coaptation of the cusps during diastole, resulting in relative valve incompetence. Causes include:
- Marfan syndrome: A hereditary connective tissue disorder affecting bones, joints, eyes, heart, and blood vessels. Typical features include long limbs, ligament and joint hypermobility, lens dislocation, and fusiform aneurysmal dilation of the ascending aorta.
- Syphilitic aortitis: Inflammation damages the aortic media, causing root dilation, with AR occurring in 30% of cases.
- Other causes, such as hypertensive aortic annular dilation, idiopathic ascending aortic dilation, aortic dissection, ankylosing spondylitis, and psoriatic arthritis.
Pathophysiology
Acute Diastolic Phase
During diastole, blood regurgitates from the aorta into the left ventricle (LV), causing a rapid increase in left ventricular end-diastolic pressure (LVEDP).
Systole
The LV struggles to eject both the regurgitant blood and the blood returning from the left atrium, leading to reduced forward stroke volume.
Diastole
The rapid rise in LV pressure causes premature closure of the mitral valve, which helps prevent excessive LV pressure elevation but limits left atrial emptying. This increases left atrial pressure, leading to pulmonary congestion and pulmonary edema.
Compensatory Mechanism
Tachycardia may compensate for the reduced forward stroke volume, maintaining systolic and aortic pressures. However, in acute AR, blood pressure often drops significantly, and cardiogenic shock may occur.
Chronic Diastolic Phase
In chronic AR, large volumes of blood regurgitate into the LV during diastole, leading to an increase in LV end-diastolic volume.
LV Dilation
The LV adapts to the chronic volume overload by dilating, allowing LVEDP to remain normal. This dilation, operating on the ascending limb of the Frank-Starling curve, enhances myocardial contractility.
Aortic Pressure Decline
The regurgitant blood flow reduces aortic pressure, which further aids LV ejection.
Normal Pulmonary Pressures
Since LVEDP remains normal, left atrial and pulmonary venous pressures are also maintained within normal limits, preventing pulmonary congestion for many years.
Disease Progression
With time, regurgitant volume increases, causing further LV dilation. LV end-diastolic volume and pressure rise significantly, eventually leading to reduced myocardial contractility, decreased stroke volume, and impaired LV function. As LV function deteriorates, left heart failure develops. LV hypertrophy increases myocardial oxygen demand, while reduced diastolic aortic pressure decreases coronary perfusion, resulting in myocardial ischemia and accelerating the decline in cardiac function.
Clinical Manifestations
Symptoms
Chronic AR can remain asymptomatic for a prolonged period, with mild cases often lasting over 20 years without symptoms. As the regurgitant volume increases, symptoms related to increased stroke volume may appear, including palpitations, precordial discomfort, and a strong pulsation in the head and neck. Symptoms of heart failure initially manifest as exertional dyspnea. As the condition progresses, paroxysmal nocturnal dyspnea and orthopnea may develop. Angina is less common compared to aortic stenosis, and syncope is rare, though dizziness or vertigo may occur with positional changes.
Acute AR may present with no symptoms in mild cases. In severe cases, symptoms can include sudden onset of dyspnea, inability to lie flat, diaphoresis, frequent coughing, and expectoration of white or pink frothy sputum. In extreme cases, patients may exhibit restlessness, confusion, or even coma.
Signs
Chronic AR
Apical impulse is displaced downward and to the left, with a broad area of pulsation. Cardiac borders are enlarged downward and to the left. Systolic thrill may be palpable at the base of the heart, the sternal notch, and carotid arteries. Carotid pulsations are markedly exaggerated.
First heart sound (S1) is diminished due to excessive left ventricular filling during diastole and elevated mitral valve position. Second heart sound (S2)is reduced or absent in the aortic valve area. Third heart sound (S3) is commonly heard at the apex, associated with rapid early diastolic filling of the left ventricle.
A high-pitched, decrescendo, blowing murmur occurs in early diastole. It is best heard at the end of expiration with the patient leaning forward, radiating to the apex. In mild AR, the murmur is soft, high-pitched, and limited to early diastole, audible only with the patient in the sitting, leaning-forward position during end-expiration. In moderate-to-severe AR, the murmur is holodiastolic and harsher in quality. A musical murmur suggests valve prolapse, rupture, or perforation. In severe AR, a mid-systolic murmur may be heard in the aortic area, radiating to the neck and suprasternal notch, which is caused by a large stroke volume passing through the deformed aortic valve, not due to organic aortic stenosis.
A soft, low-pitched, rumbling diastolic murmur (Austin-Flint murmur) can be heard at the apex. Its mechanisms include:
- Increased left ventricular volume and diastolic pressure from AR push the anterior mitral leaflet into a higher position, causing relative mitral stenosis.
- The regurgitant aortic flow collides and mixes with the mitral inflow, creating turbulence and generating the murmur.
Increased systolic blood pressure, reduced diastolic blood pressure, and widened pulse pressure cause peripheral vascular signs.
Peripheral vascular signs may include:
- De Musset's sign: Head bobbing with each heartbeat
- Water-Hammer pulse: A bounding and collapsing pulse
- Traube's sign: Pistol-shot sounds over the femoral artery
- Quincke's sign: Capillary pulsations visible in the nail beds
- Duroziez's sign: A double murmur heard over the femoral artery when compressed by a stethoscope
Acute AR
Severe acute AR may present with grayish skin, cyanosis of the lips and nail beds, tachycardia, and hypotension, indicative of shock.
S1 is diminished or absent due to premature mitral valve closure. Pulmonary hypertension may cause accentuation of the pulmonary component of S2. Pathological S3 and S4 sounds may be heard.
Diastolic murmur is soft, short, and low-pitched due to the rapid rise in left ventricular diastolic pressure, which reduces the pressure gradient between the aorta and left ventricle. Peripheral vascular signs are not prominent. Wheezing or fine crackles may be heard at the lung bases. In severe cases, diffuse crackles may be present throughout the lungs.
Laboratory and additional examinations
Chest X-ray
Chronic AR presents with marked left ventricular enlargement, with downward and leftward displacement. The cardiac silhouette appears boot-shaped, with a deepened cardiac waist and dilation of the ascending aortic arch.
Acute AR presents with normal cardiac size or slight left atrial enlargement. Pulmonary congestion and pulmonary edema are often present.
Electrocardiography (ECG)
Chronic AR
Left ventricular hypertrophy with strain and left axis deviation are common. Myocardial damage may be indicated by intraventricular conduction delays or atrial and ventricular arrhythmias.
Acute AR
Sinus tachycardia and nonspecific ST-T changes are typical.
Echocardiography
M-mode echocardiography shows rapid, high-frequency diastolic fluttering of the anterior mitral leaflet.
Two-dimensional echocardiography demonstrates incomplete closure of the aortic valve during diastole.
Doppler echocardiography detects holodiastolic regurgitant flow below the aortic valve (in the left ventricular outflow tract). This is a highly sensitive and accurate method for diagnosing AR, correlating closely with angiography, and allows quantification of severity.
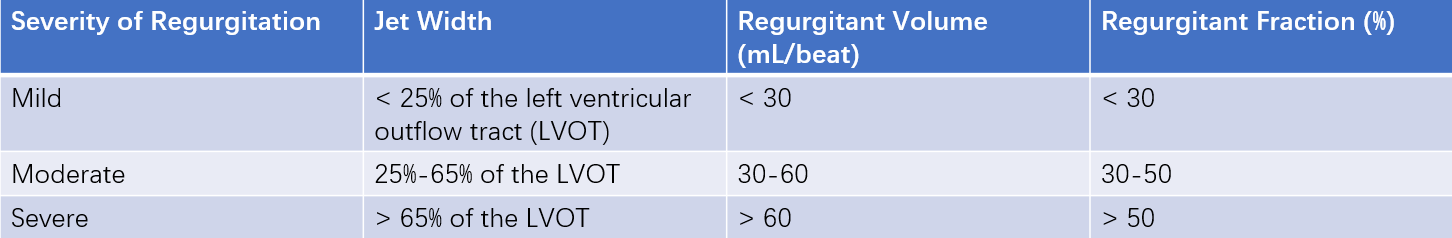
Table 1 Determination of aortic regurgitation severity
Cardiac Magnetic Resonance Imaging (MRI)
If echocardiographic assessment is suboptimal or inconclusive, cardiac MRI can be used to quantify regurgitant severity, measure left ventricular systolic and diastolic volumes, and evaluate left ventricular systolic function. MRI can also quantify regurgitant volume and regurgitant orifice area.
Cardiac Catheterization
When non-invasive techniques fail to determine the severity of regurgitation, or when surgical intervention is being considered, cardiac catheterization can be performed to assess regurgitation severity and evaluate coronary artery status.
Diagnosis
The presence of a characteristic diastolic murmur of AR accompanied by peripheral vascular signs can confirm the diagnosis of AR. Echocardiography provides definitive confirmation. Chronic AR with concurrent aortic stenosis or mitral valve disease suggests rheumatic heart disease.
Differential Diagnosis
When the murmur of AR is most prominent along the left sternal border, it should be differentiated from the Graham-Steell murmur.
The Austin-Flint murmur should be distinguished from the mid-to-late diastolic murmur of mitral stenosis at the apex. The Austin-Flint murmur typically follows the third heart sound and is associated with a diminished first heart sound. The mitral stenosis murmur follows an opening snap and is associated with an accentuated S1.
Complications
Infective endocarditis is common in AR and often accelerates the onset of heart failure.
In chronic AR, congestive heart failure typically develops in the late stage. In acute AR, congestive heart failure occurs much earlier.
Ventricular arrhythmias are common, but sudden cardiac death is rare.
Treatment
Chronic AR
General Management
Patients with chronic AR should avoid heavy physical exertion and intense exercise.
Follow-up echocardiographic evaluations are recommended:
- Mild AR: Every 3-5 years
- Moderate AR: Every 1-2 years
- Severe AR: Every 6-12 months, with increased frequency if progressive left ventricular dilation is observed
Medical Therapy
Infective endocarditis prevention is necessary for all patients. For rheumatic heart disease, prophylaxis against rheumatic fever is required.
Syphilitic aortitis requires full-course penicillin treatment.
Aggressive hypertension control is recommended using ACE inhibitors (ACEIs), angiotensin receptor blockers (ARBs), or dihydropyridine calcium channel blockers.
In patients with heart failure who are unsuitable for surgery, ACEIs, ARBs, or dihydropyridine calcium channel blockers can be used.
In cases of postoperative heart failure or hypertension, beta-blockers, ACEIs, or ARBs can be used.
In patients with Marfan syndrome, beta-blockers should be used both preoperatively and postoperatively.
In cases of bicuspid aortic valve with ascending aorta or aortic root dilation, ACEIs, ARBs, or beta-blockers are recommended.
Surgical Treatment
Patients with chronic AR who are asymptomatic and have normal LV function do not require surgery but should be monitored regularly.
Surgical intervention remains the first-line treatment for severe AR, with indications including:
- Symptomatic severe AR, regardless of LV function
- Asymptomatic severe AR with any of the following:
- Left ventricular ejection fraction (LVEF) < 55%
- Normal LVEF but with LV end-systolic diameter (LVESD) > 50 mm or LVESD index > 25 mm/m2
- Moderate or greater AR requiring other cardiac surgeries
Contraindications for surgery include:
- LVEF ≤ 15-20%
- LV end-diastolic diameter (LVEDD) ≥ 80 mm or LV end-diastolic volume index (LVEDVI) ≥ 300 mL/m2
Surgical Options
Aortic valve replacement (AVR) is the primary surgical treatment for AR. Aortic valve repair may be considered in select younger patients with suitable valve anatomy (e.g., AR due to aortic root dilation without valve thickening, deformation, or calcification).
Transcatheter aortic valve replacement (TAVR) is not currently a first-line treatment for severe AR. This is primarily because AR often lacks valve calcification, making valve positioning and fixation challenging. However, TAVR may be considered in patients with contraindications to surgery and favorable anatomical conditions at experienced centers.
Acute AR
Acute AR is significantly more dangerous than chronic AR, and surgical treatment should be considered as early as possible.
Medical treatment is generally used as a transitional measure for preoperative preparation and includes oxygen therapy, sedation, and intravenous administration of dopamine or dobutamine, or sodium nitroprusside and furosemide. Treatment should ideally be conducted under hemodynamic monitoring with a Swan-Ganz catheter at the bedside, with the primary goals of reducing pulmonary venous pressure, increasing cardiac output, and stabilizing hemodynamics. Aortic valve replacement or aortic valve repair is the definitive treatment for acute aortic regurgitation.
Prognosis
Acute severe AR is often fatal due to left heart failure if not treated surgically in time.
Chronic AR has a prolonged asymptomatic phase, but once symptoms appear, the condition deteriorates rapidly.
The annual mortality rate for patients with NYHA Class II heart failure and severe AR is approximately 6%.
For patients with NYHA Class III or IV symptoms, the annual mortality rate approaches 25%.
Most patients experience significant clinical improvement following aortic valve replacement. The heart size decreases, left ventricular mass is reduced, and LV function partially recovers. However, the degree of recovery and long-term survival are lower compared to patients with aortic stenosis.