Etiology and Pathology
The mitral valve structure consists of four components: leaflets, annulus, chordae tendineae, and papillary muscles. Normal mitral valve function depends on the integrity of these components as well as the structure and function of the left ventricle. Structural abnormalities or functional disorders in one or more of these components can lead to mitral regurgitation (MR).
Based on the disease course, MR can be classified into acute MR and chronic MR. Chronic MR is further divided into chronic primary (degenerative) MR and chronic secondary (functional) MR. Chronic primary MR results from pathological changes in one or more of the mitral valve components (leaflets, annulus, chordae tendineae, or papillary muscles). Chronic secondary MR is caused by structural or functional abnormalities of the left ventricle or left atrium, with the mitral valve itself typically being normal.
Leaflets
Approximately 30% of MR cases are caused by rheumatic damage. Rheumatic changes make the leaflets stiff and degenerated, with curling and contraction of leaflet edges, commissural fusion, and chordal fusion or shortening. Other causes include leaflet perforation and vegetation attachment due to infective endocarditis, which affects valve closure. Primary myxomatous degeneration of the mitral valve causes leaflet redundancy or elongation of the chordae tendineae, leading to mitral valve prolapse (MVP). During systole, the prolapsed leaflets bulge into the left atrium, causing MR. MVP can also occur in hereditary connective tissue disorders such as Marfan syndrome. In hypertrophic obstructive cardiomyopathy, systolic anterior motion (SAM) of the anterior mitral leaflet can result in MR. Congenital heart diseases, such as endocardial cushion defects, are often associated with clefts in the anterior mitral leaflet, leading to regurgitation.
Annular Dilation
Any condition causing left ventricular or left atrial enlargement, or degenerative changes and calcification of the mitral annulus, can lead to annular dilation and MR. Degenerative changes and calcification of the mitral annulus are more common in older females. Autopsy studies show that the incidence of mitral annular calcification in females over 70 years old is 12%. Severe mitral annular calcification is associated with a 50% prevalence of aortic annular calcification, and approximately 50% of cases involve the conduction system, leading to varying degrees of atrioventricular or intraventricular conduction block.
Chordae Tendineae
Chordae tendineae abnormalities are an important cause of MR. Congenital abnormalities, spontaneous rupture, or rupture secondary to infective endocarditis or rheumatic fever can all lead to MR.
Papillary Muscles
Dysfunction of the papillary muscles weakens their tethering effect on the chordae tendineae and leaflets, resulting in MR. The blood supply to the papillary muscles comes from the terminal branches of the coronary arteries, making them highly sensitive to ischemia. Insufficient coronary perfusion can cause ischemia, injury, necrosis, and fibrosis of the papillary muscles, leading to dysfunction. Transient papillary muscle ischemia can cause temporary MR, while acute myocardial infarction with papillary muscle necrosis can result in permanent MR. Papillary muscle necrosis is a common complication of myocardial infarction, but papillary muscle rupture occurs in less than 1% of cases. Complete rupture of the papillary muscle can lead to severe, life-threatening acute MR. Rare diseases affecting the papillary muscles include congenital anomalies (e.g., parachute mitral valve syndrome caused by the absence of one papillary muscle), abscesses, granulomas, amyloidosis, and sarcoidosis.
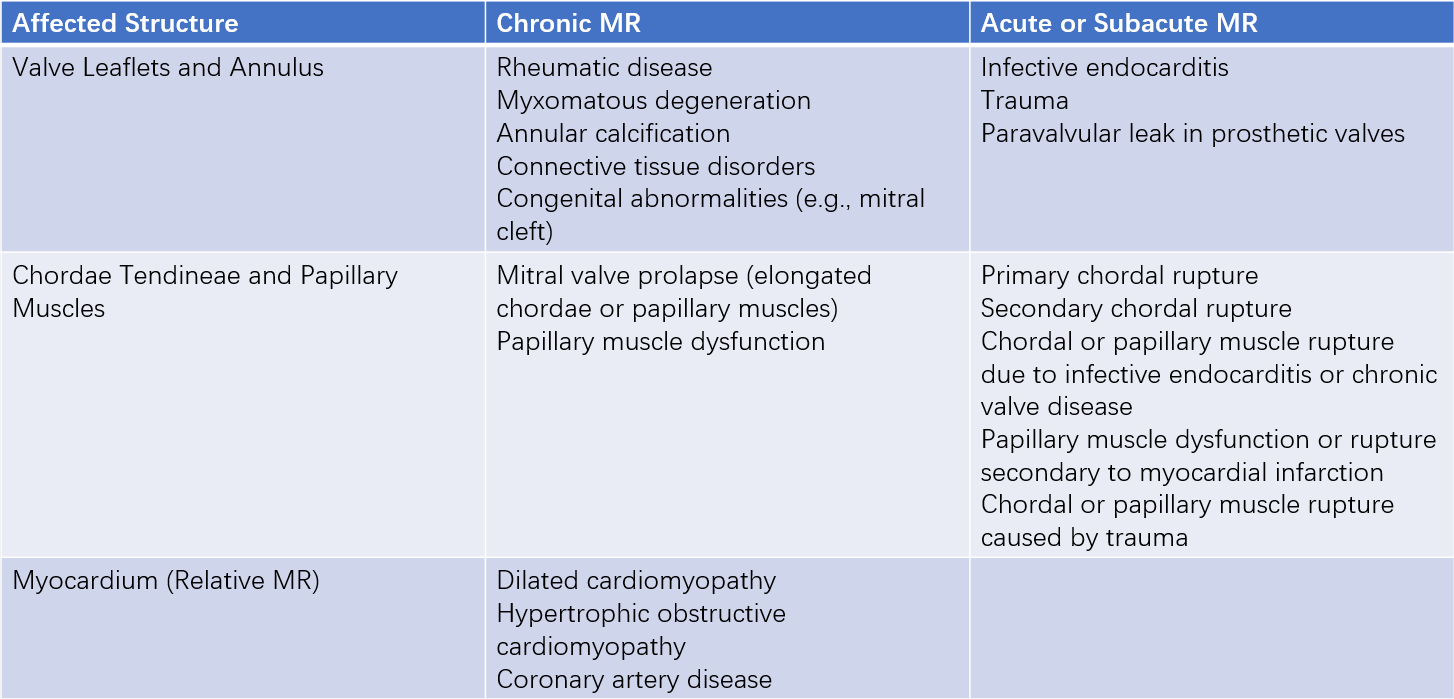
Table 1 Classification of mitral regurgitation etiologies
Pathophysiology
Acute Mitral Regurgitation
Acute MR results in a sudden increase in left atrial and left ventricular pressures, leading to pulmonary congestion and even pulmonary edema. Additionally, decreased stroke volume and cardiac output cause systemic vascular resistance to rise, further exacerbating the severity of MR. Patients typically present with sudden-onset pulmonary edema and cardiogenic shock.
Chronic Mitral Regurgitation
In chronic MR, regurgitant blood during diastole re-enters the left ventricle through the mitral valve, increasing left ventricular volume overload. Initially, the left ventricle compensates by dilating and undergoing eccentric hypertrophy. According to the Frank-Starling mechanism, this increases left ventricular stroke volume to maintain normal forward cardiac output. In chronic MR, left atrial compliance increases, leading to left atrial enlargement and adaptation of both the left atrium and left ventricle to the increased volume load over time. As a result, left atrial pressure and left ventricular end-diastolic pressure remain relatively unchanged for a prolonged period, preventing pulmonary congestion and clinical symptoms.
However, persistent and severe volume overload eventually leads to left ventricular myocardial dysfunction, causing a significant rise in left ventricular end-diastolic pressure and left atrial pressure. This results in pulmonary congestion, pulmonary hypertension, and ultimately right heart failure.
Clinical Manifestations
Symptoms
Acute Mitral Regurgitation
Mild acute MR may only cause slight exertional dyspnea. However, severe MR (e.g., due to chordae tendineae or papillary muscle rupture) can rapidly lead to acute left heart failure, presenting with acute pulmonary edema or cardiogenic shock.
Chronic Mitral Regurgitation
The severity and progression of symptoms in chronic MR depend on the degree of regurgitation, the speed of progression, left atrial and pulmonary venous pressures, pulmonary artery pressure, and the presence of other valvular lesions or coronary artery disease.
Mild MR may remain asymptomatic throughout a patient’s lifetime. In more severe cases, symptoms may take over 20 years to develop after the onset of rheumatic fever, but once heart failure occurs, it often progresses rapidly.
Patients with significant MR may experience reduced cardiac output, leading to fatigue, weakness, and decreased exercise tolerance. Pulmonary venous congestion can cause varying degrees of dyspnea, including exertional dyspnea, resting dyspnea, paroxysmal nocturnal dyspnea, and orthopnea. In advanced stages, signs of systemic venous congestion due to right heart failure may develop. Interestingly, symptoms of left heart failure may lessen after the onset of right heart failure.
Additionally, patients with concomitant coronary artery disease may present with angina symptoms due to reduced cardiac output.
Signs
Acute Mitral Regurgitation
Apical impulse is hyperdynamic. Pulmonary valve second heart sound is accentuated. A fourth heart sound (S4) may be heard at the apex due to forceful left atrial contraction. A systolic murmur, the hallmark of MR, is heard at the apex. This is typically a coarse, blowing murmur graded ≥3/6. When chordae tendineae or papillary muscles are involved, a musical murmur may be present. The duration of the regurgitant murmur is shortened and ends before the second heart sound due to reduced pressure difference between the left atrium and ventricle. Severe MR may also present with a third heart sound (S3) and a short, rumbling diastolic murmur at the apex.
Chronic Mitral Regurgitation
Apical impulse is displaced downward and laterally, with a lifting quality. In severe MR, the first heart sound (S1) is diminished or absent. However, in cases of MR caused by mitral valve prolapse or coronary artery disease, S1 may remain normal. Left ventricular ejection time is shortened, causing an early second heart sound (S2) and widened splitting. The classic murmur of MR is a holosystolic, blowing murmur heard best at the apex, graded ≥3/6, and may be accompanied by a thrill. In anterior leaflet pathology, the murmur radiates to the left axilla, subscapular region, or back, while in posterior leaflet pathology, it radiates to the base of the heart. The intensity of the murmur correlates with left ventricular contractility but does not necessarily reflect the severity of regurgitation. Rheumatic MR often coexists with the typical murmur of mitral stenosis. Mitral valve prolapse may produce a mid-systolic click. Chordal rupture can result in a murmur resembling a seagull's cry or a musical murmur.
Laboratory and Ancillary Tests
Chest X-ray
In acute MR, the cardiac silhouette is normal or shows mild left atrial enlargement with significant pulmonary congestion or even pulmonary edema.
In chronic severe MR, left atrial and left ventricular enlargement is evident. Pulmonary congestion and interstitial pulmonary edema may be seen in left heart failure.
Mitral annular calcification appears as a dense, coarse C-shaped opacity in the lateral or right anterior oblique views.
Electrocardiogram (ECG)
In acute MR, the ECG is typically normal, often showing sinus tachycardia.
In chronic severe MR, P waves may be widened and notched, indicating left atrial enlargement.
Some cases may show left ventricular hypertrophy and nonspecific ST-T changes, while a few may show right ventricular hypertrophy. Atrial fibrillation is common.
Echocardiography
M-mode echocardiography shows increased EF slope of the anterior mitral leaflet, with enlargement of the left atrium, left ventricle, and right ventricle.
Two-dimensional echocardiography demonstrates morphological changes in the mitral valve, such as leaflet and subvalvular thickening, fusion, shortening, calcification, leaflet prolapse, flail leaflet, annular dilation or calcification, vegetations, left ventricular enlargement, and paradoxical wall motion. These findings help identify the etiology of MR.
Pulsed-wave, continuous-wave, or color Doppler imaging can detect systolic regurgitant jets and regurgitation spectra in the left atrium. The sensitivity of Doppler echocardiography in diagnosing MR approaches 100%.
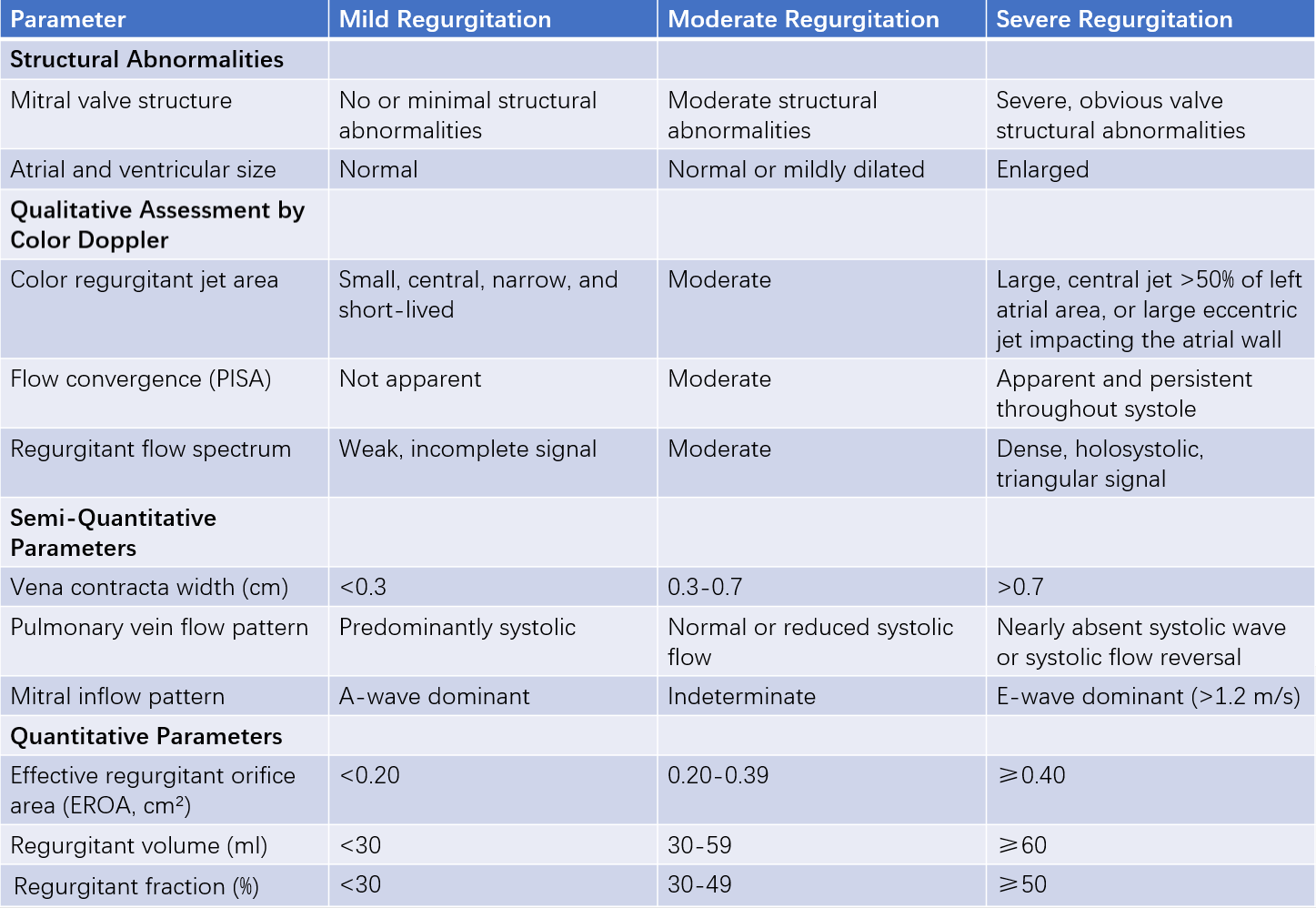
Table 2 Grading criteria for mitral regurgitation severity
Diagnosis and Differential Diagnosis
The diagnosis of MR is primarily based on the presence of a characteristic holosystolic blowing murmur at the apex and echocardiographic findings. Echocardiography helps differentiate MR from physiological murmurs, tricuspid regurgitation, ventricular septal defects, and left or right ventricular outflow tract obstruction.
Complications
Complications include:
- Atrial fibrillation (AF): Common in MR
- Infective endocarditis (IE): More frequent in MR compared to mitral stenosis
- Systemic embolism: Less common than in mitral stenosis
- Heart failure (HF): Occurring early in acute MR and later in chronic MR
- Acute left heart failure or pulmonary edema: Possibly in acute MR
Treatment
Acute Mitral Regurgitation
The goal of treatment is to reduce pulmonary venous pressure, increase cardiac output, and address the underlying cause. Medical therapy is generally a temporary measure before surgery and is guided by hemodynamic monitoring using a Swan-Ganz catheter when possible.
Nitrates and diuretics can reduce filling pressures. Intravenous nitroprusside can dilate both arterioles and venules, reducing preload and afterload, alleviating pulmonary congestion, decreasing regurgitation, and improving cardiac output.
In cases of hypotension or hemodynamic instability, positive inotropic agents, intra-aortic balloon pump (IABP), or extracorporeal membrane oxygenation (ECMO) may be used.
Definitive treatment involves emergency or elective valve repair or replacement, depending on the etiology, severity of regurgitation, and response to medical therapy. Some patients may stabilize with medical therapy and transition into a chronic compensated phase.
Chronic Mitral Regurgitation
Asymptomatic patients may not require specific treatment for long periods. The focus is on preventing rheumatic fever and infective endocarditis, with regular follow-up.
In cases of mild MR with normal cardiac function, no routine echocardiographic follow-up is needed.
In cases of moderate MR with normal cardiac function, clinical follow-up annually and echocardiographic evaluation every 1-2 years are required.
In patients with severe MR with normal cardiac function, clinical follow-up every 6 months and echocardiographic evaluation annually are required.
If significant changes occur (e.g., new-onset AF, elevated pulmonary artery pressure, or reduced left ventricular ejection fraction), strenuous activity should be avoided, follow-up frequency should be increased, and medical, interventional, or surgical treatment should be considered as needed.
In cases of primary MR, medical therapy has limited efficacy as it does not address the underlying structural abnormality. Vasodilators are not recommended for asymptomatic patients with normal blood pressure and preserved left ventricular systolic function. Standard antihypertensive therapy is recommended for patients with primary MR and hypertension. For symptomatic patients with chronic primary MR and left ventricular ejection fraction (LVEF) <60%, medical therapy following heart failure guidelines is recommended while awaiting surgery or if surgery is contraindicated.
In cases of secondary MR, optimal medical therapy for heart failure is the cornerstone of management and includes:
- ACE inhibitors (ACEIs), angiotensin receptor blockers (ARBs), or angiotensin receptor-neprilysin inhibitors (ARNIs)
- Beta-blockers
- Sodium-glucose cotransporter-2 (SGLT2) inhibitors
- Aldosterone receptor antagonists
Diuretics may be used for symptomatic relief of volume overload.
Long-term anticoagulation is indicated for patients with chronic AF, a history of systemic embolism, or left atrial thrombus.
Surgery is the definitive treatment to restore valve function.
Indications for surgery include:
- Severe primary MR with clinical symptoms
- Asymptomatic severe primary MR with pulmonary hypertension (mean pulmonary artery pressure >50 mmHg), new-onset AF, or left ventricular dysfunction (LVEF ≤60% or left ventricular end-systolic diameter [LVESD] ≥40 mm)
- Severe secondary MR requiring concomitant coronary artery bypass grafting (CABG) or other cardiac surgeries
Cardiac catheterization and angiography are performed to assess hemodynamics, the severity of MR, and coronary artery disease, guiding surgical planning.
Mitral valve repair is preferred for non-rheumatic, non-infective, and non-ischemic etiologies, such as mitral valve prolapse, chordae tendineae rupture, and annular dilation.
Mitral valve replacement can be considered when repair is not feasible.
Mitral valve prolapse is the most common cause of isolated severe MR. Patients with severe symptoms unresponsive to medical therapy should undergo timely mitral valve repair.
Acute MR due to papillary muscle rupture, trauma, or infective endocarditis has a higher mortality rate with emergency surgery compared to elective surgery.
In cases of MR secondary to acute myocardial infarction, surgery is ideally performed 4-6 weeks after infarction if the condition stabilizes with medical therapy during this period.
Cardiac resynchronization therapy (CRT) can improve secondary MR in patients with ventricular dyssynchrony. It is recommended for eligible patients with secondary MR. Careful patient selection is required, particularly in ischemic MR, as CRT may not effectively pace scarred myocardial regions.
Transcatheter techniques are rapidly advancing and aim to improve outcomes in high-risk surgical patients with severe MR. These include:
- Transcatheter edge-to-edge repair (TEER)
- Transcatheter mitral chordal implantation
- Transcatheter mitral annuloplasty
- Transcatheter mitral valve replacement
TEER indications include:
- Symptomatic primary MR with contraindications or high surgical risk and suitable anatomy
- Symptomatic severe secondary MR despite optimal medical therapy (including CRT if indicated) and high surgical risk or contraindications
Prognosis
Acute severe MR caused by abnormalities of the papillary muscles, chordae tendineae, or valve leaflets with hemodynamic instability has a very high mortality rate without prompt surgical intervention.
For chronic severe MR, the 5-year survival rate with medical therapy is approximately 80%, and the 10-year survival rate is about 60%.
Patients with isolated mitral valve prolapse and no significant regurgitation generally have a good prognosis. However, prognosis worsens in patients over 50 years old with significant systolic murmurs, marked mitral regurgitation, elongated and thickened valve leaflets, and left atrial or left ventricular enlargement.