Infective endocarditis (IE) refers to an infectious disease caused by pathogens that infect heart valves, the endocardium, or the endothelium of adjacent large blood vessels through hematogenous dissemination or direct invasion. The infection most commonly involves the heart valves and is often accompanied by vegetation formation, localized structural and functional damage, septic embolism to distant organs, and systemic inflammatory responses.
In recent years, the incidence of IE has shown an upward trend, which is associated with an increase in degenerative valvular diseases in the elderly, implantation of cardiac devices, medical procedures, and intravenous drug use. In 2019, the global estimated incidence was 13.8 per 100,000 people per year, with a male-to-female ratio of ≥2:1. The spectrum of pathogens causing IE has also undergone significant changes, with the most common causative agents shifting from streptococci to staphylococci. Additionally, drug-resistant strains are increasingly prevalent.
IE can be classified based on various criteria: by disease severity and progression (acute and subacute); by mode of acquisition (healthcare-associated, community-acquired, or behavior-related, such as in intravenous drug users); and by the type of affected valve (native valve endocarditis [NVE] and prosthetic valve endocarditis [PVE]).
Etiology
Predisposing Factors
These include underlying heart diseases (such as valvular diseases, especially mitral valve prolapse, and congenital heart diseases), immunocompromised conditions (such as AIDS, immunosuppression, and diabetes), cardiac device implantation (such as pacemakers and prosthetic valves), medical procedures (such as hemodialysis, intravascular catheterization, and dental treatments), and personal behaviors (such as intravenous drug use and tattooing).
Infectious Pathogens
These include bacteria, fungi, viruses, rickettsiae, and chlamydiae. Approximately 90% of community-acquired IE cases are caused by streptococci, staphylococci, or enterococci (with streptococci being the most common). Healthcare-associated or intravenous drug use-related IE is often caused by Staphylococcus aureus. Acute IE is primarily caused by Staphylococcus aureus, while subacute IE is often due to viridans streptococci.
Pathogenesis
Experimental models suggest that IE follows a predictable sequence: endothelial injury → platelet and fibrin aggregation forming sterile vegetations → colonization of sterile vegetations by pathogens during bacteremia → formation of infected vegetations → embolization of vegetation fragments and systemic dissemination. Similarly, the clinical course of typical subacute IE follows this process, whereas acute IE has a more abrupt onset and can rapidly lead to valve destruction and death in a short period.
Pathology
Cardiac Lesions
Vegetation Formation
Vegetation is the most characteristic pathological feature. It may appear verrucous, cauliflower-like, or polypoid in shape, with varying sizes and high mobility, occasionally leading to obstruction of the valve orifice.
Invasive Lesions
The infection may directly cause valve destruction and chordae tendineae rupture, resulting in valvular insufficiency. The infection may also invade adjacent structures, leading to complications such as perivalvular abscesses, myocardial or pericardial suppuration, conduction block, and septal perforation.
Extracardiac Lesions
Systemic Embolism
Embolization of vegetation fragments from the left heart system can lead to infarction or abscess formation in terminal organs such as the brain, kidneys, heart, intestines, and peripheral vessels.
Pulmonary Embolism
Embolization of vegetation fragments from the right heart system can result in pulmonary infarction or lung abscesses.
Metastatic Abscesses
These occur when bacteremia disseminates pathogens to extracardiac sites.
Vascular Wall Damage
Septic emboli can occlude or damage the arterial vasa vasorum or lumen, causing vascular wall necrosis and aneurysm formation.
Immune-Mediated Damage
Repeated stimulation of cellular and humoral immunity by bacteremia can lead to complications such as splenomegaly, glomerulonephritis, arthritis, pericarditis, and vasculitis.
Clinical Manifestations
The onset of infective endocarditis (IE) can be either acute or subacute. Acute IE typically has a rapid onset, progresses quickly (leading to valve destruction within days), presents with significant symptoms, and frequently involves metastatic infections, with Staphylococcus aureus being the most common pathogen. Subacute IE progresses more slowly, with an onset over weeks to months, milder symptoms, and fewer metastatic infections, often caused by viridans streptococci, followed by enterococci. Clinically, the two forms often overlap, making them difficult to distinguish completely. The main clinical syndromes include the following:
Infectious Symptoms
Fever occurs in almost all patients, with a typical remittent fever pattern, generally below 39°C. Subacute cases often present with an insidious onset, featuring prolonged, irregular low-grade fever accompanied by fatigue, loss of appetite, diaphoresis, and emaciation, which are nonspecific symptoms. Acute cases are characterized by a fulminant septic process, with high fever, rigors, headache, back pain, and musculoskeletal pain.
Cardiac Murmurs
Cardiac murmurs are present in up to 85% of patients and may result from underlying heart disease and/or newly developed valvular damage. An increase in murmur intensity, changes in murmur characteristics (e.g., a seagull’s cry murmur), or the appearance of a new murmur is highly suggestive of valvular damage leading to regurgitation. These findings are more common in acute cases, particularly in aortic valve IE.
Peripheral Signs
Peripheral signs are now rare and nonspecific, potentially resulting from immune-mediated microvasculitis or microembolism. These may include:
- Petechiae: Found in any location but most commonly on the skin above the clavicles, oral mucosa, and conjunctiva, and more frequent in prolonged cases.
- Subungual Splinter Hemorrhages: Linear hemorrhages under the nails.
- Roth Spots: Oval retinal hemorrhages with pale centers, more common in subacute cases.
- Osler Nodes: Painful, red or purple, pea-sized nodules on the finger or toe pads, more frequent in subacute cases.
- Janeway Lesions: Painless hemorrhagic macules, 1-4 mm in diameter, on the palms or soles, more common in acute cases.
Arterial Embolism
Symptomatic arterial embolism occurs in approximately 25% of cases, with asymptomatic cases being more common. Vegetations from the left heart system primarily cause systemic embolism, potentially affecting the brain, heart, kidneys, spleen, mesentery, and extremities, with cerebral embolism being the most common (15-20%). Vegetations from the right heart system primarily result in pulmonary embolism.
Other Symptoms
Other nonspecific manifestations include splenomegaly, anemia, and clubbing of the fingers.
Complications
Acute IE has a high rate of complications, which are the main causes of mortality and morbidity.
Cardiac Complications
Cardiac complications are present in the vast majority of cases and include:
- Heart Failure: The most common complication, often caused by valve perforation or chordae tendineae rupture, leading to acute valvular regurgitation. The aortic valve is most frequently affected, followed by the mitral and tricuspid valves.
- Myocardial Abscess: Often occurs around the aortic valve and may lead to conduction block or purulent pericarditis.
- Myocardial Infarction: Detected in up to 50% of autopsies, though often asymptomatic. It is more common in aortic valve IE and typically caused by septic emboli.
- Myocardial Inflammation: Less common.
Neurological Complications
Neurological complications are common but often asymptomatic, with only 15-30% of patients showing clinical signs of brain involvement:
- Cerebral Embolism: Occurs in about half of cases, most commonly affecting the middle cerebral artery and its branches.
- Mycotic Aneurysms: Often asymptomatic unless rupture occurs, leading to hemorrhage.
- Cerebral Hemorrhage: Caused by hemorrhagic transformation of embolic infarcts or rupture of mycotic aneurysms.
- Toxic Encephalopathy, Purulent Meningitis, and Brain Abscess: More common in acute Staphylococcus aureus IE.
Renal Complications
Renal involvement is common and includes:
- Renal Infarction: Detected in ≥50% of autopsies, though often asymptomatic.
- Focal/Diffuse Glomerulonephritis: Immune complex-mediated and more common in subacute cases.
- Renal Abscess: Rare.
Splenic Complications
Splenic infarction is found in 44% of autopsies, though often asymptomatic. Some patients may experience left upper quadrant pain radiating to the left shoulder.
Pulmonary Complications
Right-sided IE frequently results in pulmonary embolism, which can progress to pulmonary necrosis, cavitation, or even pyopneumothorax.
Vascular Complications
Mycotic aneurysms occur in 3-5% of cases, more commonly in subacute IE. The most commonly affected arteries are the proximal aorta and sinuses, followed by cerebral, visceral, and peripheral arteries.
Auxiliary Examinations
Routine Laboratory Tests
Urinalysis
Microscopic hematuria and proteinuria are common findings. Gross hematuria suggests renal infarction. The presence of red blood cell casts and significant proteinuria indicates diffuse glomerulonephritis.
Blood Tests
Subacute cases often present with normochromic normocytic anemia, normal or mildly elevated white blood cell counts, and mild left shift. Acute cases are typically associated with leukocytosis and a marked left shift. The erythrocyte sedimentation rate (ESR) is almost always elevated.
Immunological Tests
Hypergammaglobulinemia is observed in 25% of cases, circulating immune complexes in 80%, and rheumatoid factor positivity in 50%. Decreased serum complement levels are seen in cases with diffuse glomerulonephritis.
Blood Cultures
Blood culture is the primary diagnostic method for IE. To ensure the quality of blood cultures, specific protocols are followed:
Collection Method
For subacute cases without prior antibiotic treatment, blood should be drawn three times on the first day, one hour apart. If no bacterial growth is observed the next day, an additional three sets of cultures should be obtained before starting antibiotic treatment. For patients already on antibiotics, discontinuation of antibiotics for 2-7 days is recommended, if the clinical condition allows, before collecting blood cultures. For acute cases, blood should be drawn immediately upon admission, three times at one-hour intervals, followed by the initiation of antibiotic therapy.
Key Considerations
Persistent bacteremia is characteristic of IE, so blood cultures can be performed regardless of fever. Blood should be drawn from different sites with strict aseptic techniques. A sample of 20 ml of peripheral venous blood should be divided equally for aerobic and anaerobic cultures, which should be incubated for at least three weeks, with periodic Gram staining and subcultures. If bacteria are detected, repeat blood cultures should be performed in 2-3 days to assess treatment efficacy.
Culture-Negative Cases
Blood culture negativity occurs in 2.5-31% of cases. The most common cause is recent or ongoing antibiotic use, followed by fungal infections, and, less commonly, infections with fastidious or atypical pathogens. For patients on antibiotics, discontinuation and repeat blood cultures may be considered if the clinical condition allows. Fungal cultures should be performed if fungal infection is suspected, particularly in patients with prosthetic valves, implanted cardiac devices, central venous catheters, urinary catheters, or intravenous drug use. For suspected fastidious or atypical pathogens, serological testing should be conducted based on local epidemiological data, targeting pathogens such as Coxiella burnetii (Q fever), Bartonella, Aspergillus, Mycoplasma pneumoniae, Brucella, and Legionella pneumophila. Specific polymerase chain reaction (PCR) testing can be used to detect Tropheryma whipplei, Bartonella, and fungi in blood or tissue samples. Additionally, metagenomic next-generation sequencing (mNGS) can provide rapid pathogen identification without the need for culture.
Electrocardiography (ECG)
Acute myocardial infarction and new-onset conduction block are occasionally observed. The latter suggests the presence of an aortic valve ring or interventricular septal abscess.
Imaging Studies
Imaging findings are critical for the diagnosis of IE, guiding treatment decisions, evaluating therapeutic efficacy, and assessing prognosis. Commonly used imaging modalities include echocardiography (UCG), computed tomography (CT), single-photon emission computed tomography (SPECT), positron emission tomography (PET), and magnetic resonance imaging (MRI).
Echocardiography (UCG)
Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) can detect vegetations, valvular damage (e.g., perforation, chordae rupture, regurgitation), perivalvular lesions (e.g., abscesses, fistulas), and prosthetic valve dehiscence. The size (length) of vegetations is a key indicator for predicting complications and making surgical decisions. TEE is more sensitive than TTE for detecting vegetations, with sensitivities of 96% and 75%, respectively, for native valve endocarditis (NVE), and 92% and 50%, respectively, for prosthetic valve endocarditis (PVE). Both have a specificity of approximately 90%. TEE is particularly advantageous for detecting vegetations smaller than 3 mm, vegetations attached to prosthetic valves or implanted devices, and in cases with poor transthoracic acoustic windows. When TTE results are negative or inconclusive, TEE or repeated examinations are recommended.
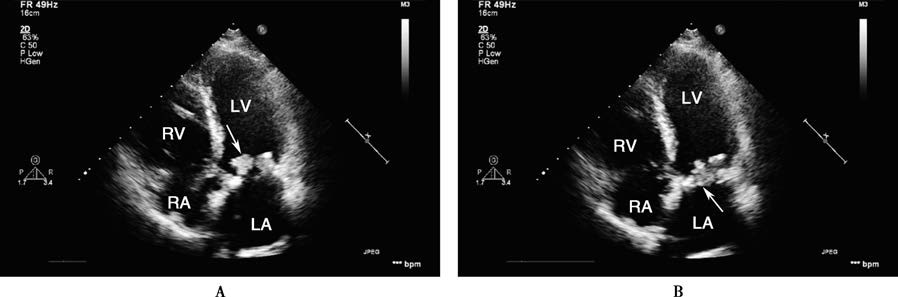
Figure 1 Transthoracic echocardiographic findings of infective endocarditis
This case involves a 53-year-old male patient who underwent mitral bioprosthetic valve replacement. In the apical five-chamber view, the bioprosthetic valve appears thickened, with a cauliflower-like vegetation attached to the anterior leaflet. During diastole (A), the vegetation protrudes into the left ventricle, while during systole (B), it returns to the valve orifice (indicated by the arrow). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Computed Tomography (CT)
CT is used for the following purposes:
- Diagnosis of IE and Cardiac Complications: CT is superior to TEE in detecting perivalvular lesions but less effective for vegetations smaller than 10 mm, valve perforations, and fistulas.
- Detection of Distant Lesions and Sources of Bacteremia: Whole-body and brain CT can evaluate systemic complications of IE. CT angiography can identify mycotic aneurysms in any part of the vascular tree. Whole-body CT can also detect extracardiac sources of bacteremia. CT findings have been incorporated into the major and minor diagnostic criteria for IE, aiding in diagnosis and treatment planning. Although MRI is superior to CT for detecting neurological complications, CT is more practical in emergency situations.
Magnetic Resonance Imaging (MRI)
MRI serves a similar role to CT, with its main advantage being the detection of neurological and spinal complications. However, its diagnostic value for cardiac lesions and local complications is inferior to that of CT.
SPECT and PET
Imaging with 18F-FDG PET-CT or radiolabeled white blood cell SPECT-CT can detect occult intracardiac and extracardiac lesions (e.g., periprosthetic infections or extracardiac infection foci), aiding in the confirmation or exclusion of IE.
Diagnosis and Differential Diagnosis
Clues Suggesting Diagnosis
The possibility of this disease is indicated in individuals with predisposing factors who present with unexplained persistent fever, changes in existing heart murmurs or new-onset murmurs, and peripheral signs (such as petechiae, Osler nodes, or Roth spots).
Diagnostic Criteria
The culture or identification of pathogens from pathological specimens remains the gold standard for diagnosis, though it is often difficult to achieve. Blood cultures and echocardiography remain the cornerstone of clinical diagnosis for infective endocarditis (IE). Positive blood cultures obtained twice with the same pathogen and the detection of vegetations on echocardiography are the most critical diagnostic evidence. However, both methods have certain false-negative rates, necessitating a comprehensive evaluation using other indicators. The Duke criteria were previously recognized as the standard for clinical diagnosis. Building on this, the 2023 European Society of Cardiology (ESC) released updated diagnostic criteria for IE.

Table 1 2023 ESC diagnostic criteria for infective endocarditis
According to the new criteria, a definitive diagnosis requires meeting two major criteria, or one major criterion plus at least three minor criteria, or five minor criteria. A possible diagnosis is suggested by the presence of one major criterion plus one to two minor criteria, or three to four minor criteria. A diagnosis is excluded if the patient does not meet the criteria for definite or possible IE upon admission, regardless of the presence or absence of alternative diagnostic indicators.
Differential Diagnosis
This disease can involve multiple organs and systems, presenting with diverse and nonspecific clinical manifestations, requiring differentiation from many other conditions. Subacute cases should be distinguished from rheumatic fever, systemic lupus erythematosus, left atrial myxoma, lymphoma, occult infections, and tuberculosis. Acute cases should also be differentiated from various types of septicemia.
Treatment
The main treatment approaches include medical and surgical interventions. Successful treatment is marked by the eradication of pathogens through antibiotic therapy. Surgical intervention is required when infections are difficult to control or when complications occur or are likely to occur.
Antibiotic Therapy
General Principles
For patients with unknown pathogens and severe conditions, empirical therapy should be initiated promptly. For patients with identified pathogens, treatment should be tailored according to antimicrobial susceptibility and the minimum inhibitory concentration (MIC), focusing on proven targeted therapies.
Specific Principles
Specific principles include:
- Early Treatment: Treatment should begin immediately after collecting three to six sets of blood cultures.
- Combination Therapy: Successful treatment depends on bactericidal, rather than bacteriostatic, effects. Combination therapy should include two bactericidal agents with synergistic effects, targeting both actively dividing and dormant bacteria.
- Prolonged and Adequate Dosage: Treatment should follow recommended dosages, typically lasting 2-6 weeks for native valve endocarditis (NVE) and 6-8 weeks for prosthetic valve endocarditis (PVE).
- Intravenous Administration: Intravenous antibiotics are preferred to maintain stable and effective blood concentrations.
- Rational Drug Selection: Drug selection should consider local epidemiological data and drug availability. These principles aim to eliminate pathogens within vegetations, reduce recurrence, and prevent resistance. However, high-dose, long-term combination therapy increases the risk of toxic side effects, dysbiosis, and superinfections.
Empirical Therapy
Empirical therapy is suitable for patients with unknown pathogens who are critically ill and require urgent treatment. Blood cultures should be collected three times at one-hour intervals before initiating treatment. Once the pathogen is identified, empirical therapy should be adjusted to targeted therapy.
The strategy for empirical therapy aims to provide broad coverage for potential pathogens. Treatment regimens should consider patient characteristics (e.g., prior antibiotic use and its efficacy, NVE or PVE, early or late PVE), infection sources (community-acquired or healthcare-associated), and local epidemiological trends (e.g., predominant pathogens and resistance patterns). For community-acquired NVE and late PVE, the regimen should cover Staphylococcus, Streptococcus, and Enterococcus. A combination of ampicillin, β-lactamase-resistant penicillin, and gentamicin is a common option. For patients allergic to penicillins or β-lactams, cefazolin or vancomycin combined with gentamicin can be used as alternative therapies. For early PVE or healthcare-associated NVE, the regimen should cover methicillin-resistant/sensitive Staphylococcus (MRSA/MSSA) and Enterococcus, with additional coverage for non-HACEK Gram-negative organisms. A combination of vancomycin (or daptomycin), gentamicin, and rifampin is a preferred option.
Targeted Therapy
This is applicable to patients with known pathogens. Treatment strategies should be adjusted based on blood culture results, antimicrobial susceptibility testing, and minimum inhibitory concentration (MIC) results, narrowing the treatment scope appropriately and selecting validated targeted regimens.
Staphylococcus
Treatment regimens are primarily determined by methicillin sensitivity. Before susceptibility results are available, beta-lactamase-resistant penicillins combined with aminoglycosides are preferred. For methicillin-sensitive Staphylococcus aureus (MSSA), oxacillin or cefazolin monotherapy is recommended. For patients allergic to penicillins, cefazolin monotherapy or a combination of daptomycin with cefazolin or fosfomycin can be used. For methicillin-resistant Staphylococcus aureus (MRSA), vancomycin monotherapy is recommended, though a combination of daptomycin with oxacillin, cefazolin, or fosfomycin is also an option. For prosthetic valve endocarditis (PVE), vancomycin combined with rifampin and gentamicin is the first choice.
Streptococcus
Treatment regimens are primarily determined by penicillin sensitivity. For susceptible strains, penicillin G, amoxicillin, or ceftriaxone monotherapy is recommended. For resistant strains, higher doses of penicillin G, amoxicillin, or ceftriaxone combined with gentamicin are recommended. For patients allergic to beta-lactams, vancomycin monotherapy is suggested. For PVE, vancomycin combined with gentamicin is recommended.
Enterococcus
Treatment regimens are primarily determined by sensitivity to beta-lactams and aminoglycosides. For susceptible strains, ampicillin or amoxicillin combined with ceftriaxone or aminoglycosides is recommended. For strains highly resistant to aminoglycosides, ampicillin or amoxicillin combined with ceftriaxone is suggested. For patients allergic to or resistant to beta-lactams, vancomycin or teicoplanin combined with aminoglycosides is recommended. For vancomycin-resistant strains, daptomycin combined with beta-lactams or fosfomycin is recommended.
Gram-Negative Bacteria
HACEK Organisms: For non-beta-lactamase-producing strains, ampicillin combined with gentamicin is preferred. For beta-lactamase-producing strains, third-generation cephalosporins such as ceftriaxone or fluoroquinolones like ciprofloxacin are recommended.
Non-HACEK Organisms: Due to their rarity and severity, early surgical intervention or prolonged antibiotic therapy (≥6 weeks) is preferred. Drug therapy may involve a combination of beta-lactams and aminoglycosides.
Fungal Infections
Fungal endocarditis has a high mortality rate and poor response to antifungal therapy, necessitating a lower threshold for surgical intervention. For Candida infections, high-dose amphotericin B, with or without flucytosine, is recommended. For Aspergillus infections, voriconazole is the first-line treatment. Long-term or even lifelong suppressive therapy with azoles is suggested.
Surgical Treatment
Surgical intervention should be considered promptly for patients with heart failure complications, uncontrolled infections, or a high risk of embolism. The indications for surgery in native valve endocarditis (NVE) are as follows:
- Emergency Surgery (<24 Hours):
- Acute severe aortic or mitral regurgitation, obstruction, or perivalvular fistula leading to refractory heart failure, pulmonary edema, or cardiogenic shock.
- Urgent Surgery (<7 Days):
- Acute severe aortic or mitral regurgitation or obstruction causing symptomatic heart failure or hemodynamic abnormalities detected by echocardiography.
- Uncontrolled local infectious foci (e.g., abscess, pseudoaneurysm, fistula, or enlarging vegetations).
- Fungal or multidrug-resistant bacterial infections.
- Persistent positive blood cultures despite appropriate antimicrobial therapy.
- Aortic or mitral valve IE with at least one embolic event and vegetations >10 mm despite appropriate antimicrobial therapy.
- Aortic or mitral vegetations >10 mm with severe valve stenosis or regurgitation.
- Aortic or mitral IE with a single large vegetation (>30 mm).
For right-sided IE, surgical treatment is indicated for refractory infections (e.g., fungal infections), persistent bacteremia lasting >7 days despite medical therapy, recurrent pulmonary embolism with tricuspid vegetations >20 mm, or secondary right heart failure.
Prevention
The most effective measures for preventing IE include maintaining good oral hygiene and undergoing regular dental check-ups. Strict aseptic techniques are required during intravenous catheter insertion or other invasive procedures. The use of prophylactic antibiotics to prevent IE has been reduced compared to the past. For high-risk patients with predisposing factors for IE, prophylactic antibiotics may be used during procedures.
For high-risk dental procedures in at-risk patients, the primary target is oral streptococci. A single dose of antibiotics is recommended 30-60 minutes before the procedure. Options include amoxicillin or ampicillin (2 g, orally or intravenously). For patients allergic to these drugs, clindamycin (600 mg, orally or intravenously) is recommended. Other options include cefazolin, ceftriaxone, or cephalexin.
Prognosis
The prognosis for IE is poor, with an in-hospital mortality rate of 15-30%. Prognosis is primarily influenced by patient characteristics, complications, pathogens, and echocardiographic findings. The main causes of death include heart failure, renal failure, septic embolism, rupture of mycotic aneurysms, or severe infections.