The aortic wall consists of three layers: the intima, media, and adventitia. When the intima of the aorta tears due to various factors, high-velocity blood flow from the lumen enters the medial layer through the tear, extending along the longitudinal axis of the vessel and forming a pathological condition with true and false lumens. This condition is referred to as aortic dissection (AD). Aortic dissection is a critical cardiovascular emergency characterized by its acute onset and high mortality rate. If not diagnosed and treated promptly, the mortality rate can reach 50% within 48 hours. Causes of death include rupture of the dissection, progressive mediastinal or retroperitoneal hemorrhage, and acute heart failure. Some patients die before reaching the hospital, and without postmortem examination, the exact cause of death often remains undetermined, making it difficult to obtain accurate epidemiological data. It is estimated that the annual incidence of aortic dissection is approximately 2.6-3.5 per 100,000 individuals. Males account for 65-75% of cases, with a higher incidence observed in winter and spring.
Etiology, Pathology, and Pathogenesis
The etiology of aortic dissection can be categorized into three groups: genetic abnormalities, degenerative changes, and trauma. Genetic abnormalities associated with aortic dissection may occur as part of syndromic conditions, such as Marfan syndrome, Loeys-Dietz syndrome, vascular Ehlers-Danlos syndrome, or Turner syndrome. Non-syndromic causes include conditions such as bicuspid aortic valve and familial thoracic aortic aneurysm. Degenerative aortic dissection is sporadic and clinically the most common type, without any known genetic abnormalities. Traumatic aortic dissection may result from blunt trauma or iatrogenic injuries, such as those caused by intra-aortic balloon counterpulsation, cardiac valve surgeries, or aortic procedures.
Most cases of aortic dissection occur when blood enters the medial layer through a tear in the intima. In some cases, rupture of the vasa vasorum in the medial layer leads to hematoma formation, which, under high pressure, subsequently tears the intima. Intimal tears are most commonly located at the sinotubular junction or near the subclavian artery, regions that experience high shear stress. As the dissection extends proximally or distally, compression of the true lumen and malperfusion of branch vessels may result, causing ischemia of the affected organs.
The exact pathogenesis of aortic dissection remains unclear. Increased activity of matrix metalloproteinases, which degrade structural proteins in the aortic wall, is thought to be one of the mechanisms involved. Histological findings include degeneration of collagen in the medial layer, fragmentation of elastic fibers, focal loss of smooth muscle cells, vacuolar degeneration of the media filled with mucoid material, and fibrotic changes in chronic cases.
Classification and Staging
Aortic dissection can be classified based on its origin and the extent of aortic involvement. The DeBakey classification system divides aortic dissections into three types:
- Type I: The dissection originates in the ascending aorta and extends beyond the aortic arch to the descending aorta, and possibly to the abdominal aorta. This is the most common type.
- Type II: The dissection originates and is confined to the ascending aorta.
- Type III: The dissection originates in the descending aorta distal to the left subclavian artery and extends distally, potentially reaching the abdominal aorta. Type IIIa involves only the thoracic descending aorta, while Type IIIb involves both the thoracic and abdominal aorta.
The Stanford classification system categorizes aortic dissections into two types:
- Type A: Dissections involving the ascending aorta, regardless of the site of origin. This corresponds to DeBakey Type I and Type II.
- Type B: Dissections originating in the descending thoracic aorta without involvement of the ascending aorta. This corresponds to DeBakey Type III.
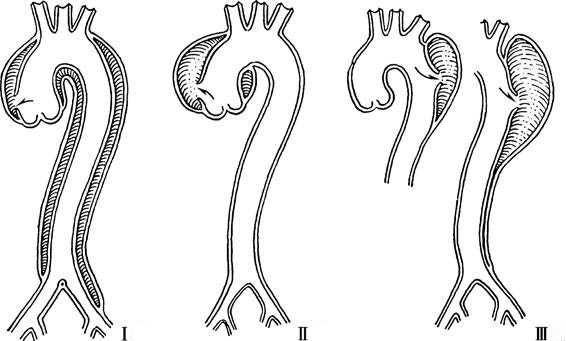
Figure 1 Schematic diagram of DeBakey classification of aortic dissection
In terms of clinical staging, aortic dissections are generally considered acute if they occur within 2 weeks after onset, subacute between 2 weeks and 3 months, and chronic if they persist beyond 3 months. Asymptomatic cases discovered incidentally during physical examinations are typically classified as chronic.
Clinical Manifestations
The clinical manifestations of aortic dissection depend on the location, extent, and severity of the dissection, involvement of aortic branches, presence of aortic valve insufficiency, and complications such as rupture into surrounding structures.
Pain
Pain is the most common and significant symptom of this condition. Over 80% of patients experience sudden, severe, continuous, tearing or knife-like pain in the anterior chest or chest and back regions. The intensity of the pain is often unbearable, and its location is closely related to the origin of the dissection. Stanford Type A dissections are often associated with chest or back pain, while Stanford Type B dissections are more commonly associated with back or abdominal pain, although overlap in pain locations exists. Dissections involving the iliac or femoral arteries may cause lower limb pain. Some patients, especially those on corticosteroid therapy or with a slow onset, may develop a dissection without significant pain.
Blood Pressure Changes
Most patients present with hypertension, often with significant differences in blood pressure between the upper limbs or between the upper and lower limbs. Hypotension may occur in cases of cardiac tamponade, hemothorax, or myocardial infarction caused by coronary artery obstruction. Rupture of the dissection with hemorrhage may result in severe shock.
Cardiovascular System
Aortic Valve Insufficiency and Heart Failure
Approximately half of patients with Stanford Type A dissections develop aortic valve insufficiency. A characteristic diastolic murmur resembling a sigh may be heard in the precordial area, and congestive heart failure may occur. However, the murmur may be less noticeable in cases of severe heart failure or tachycardia.
Myocardial Infarction
Proximal extension of the dissection may cause the intimal flap to obstruct the coronary ostia, leading to acute myocardial infarction. The right coronary artery is most commonly affected, resulting in inferior wall myocardial infarction.
Cardiac Tamponade
Details can be found in the corresponding section.
Organ or Limb Ischemia
Neurological Ischemia
Dissections involving the carotid or brachiocephalic arteries may result in cerebral ischemia, causing symptoms such as dizziness, transient syncope, mental disturbances, or, in severe cases, ischemic stroke. Extensions down to the second lumbar vertebral level may involve the anterior spinal artery, leading to paraplegia, urinary and fecal incontinence, and other symptoms.
Limb Ischemia
Dissections involving the abdominal aorta or iliac arteries may cause acute lower limb ischemia, with clinical findings such as diminished or absent pulses, cold extremities, and cyanosis.
Visceral Ischemia
Involvement of the renal arteries may lead to symptoms such as flank pain, hematuria, oliguria, anuria, or other signs of renal dysfunction. Involvement of the superior mesenteric artery may cause intestinal necrosis. Jaundice and elevated serum transaminases may indicate hepatic artery occlusion and subsequent liver ischemia.
Compression Symptoms
As the dissection progresses and forms a dissecting aneurysm, compression symptoms may occur. Compression of the cervical sympathetic ganglia may result in Horner syndrome, while compression of the left recurrent laryngeal nerve may cause hoarseness. Compression of the trachea may lead to respiratory distress, and compression of the esophagus may cause dysphagia.
Auxiliary Examinations
When aortic dissection is suspected, diagnostic methods include transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), computed tomography angiography (CTA), magnetic resonance angiography (MRA), and digital subtraction angiography (DSA). Each method has its advantages and limitations in terms of diagnostic capability, speed, convenience, and risk.
Chest X-Ray and Electrocardiography (ECG)
These methods lack specific diagnostic value. Chest X-rays may show a widened aortic silhouette. ECG findings are generally nonspecific unless there is pericardial effusion or coronary artery involvement, in which case ST-T changes may occur.
Echocardiography
Echocardiography can visualize the true and false lumens of the aortic dissection and assess blood flow, as well as detect complications such as aortic valve insufficiency and cardiac tamponade. It is non-invasive, does not require contrast agents, and can be performed at the bedside. Sensitivity ranges from 59% to 85%, and specificity ranges from 63% to 96%. However, air in the airway may interfere with imaging, leading to missed diagnoses. Transesophageal echocardiography may induce gagging, tachycardia, or hypertension and sometimes requires anesthesia, making it less commonly used in clinical practice.
Aortic CTA
Contrast-enhanced CTA is the most commonly used method for evaluating aortic dissection, with a sensitivity and specificity of 98%. Using ECG-gated techniques and thin-slice scanning with 64-slice or higher equipment reduces motion artifacts caused by cardiac pulsation. CTA can provide information on the size and location of the intimal tear, the extent of the dissection, the differentiation between true and false lumens, the perfusion status of branch vessels, and the size of vascular access routes. Limitations include side effects of contrast agents and exposure to ionizing radiation.
Aortic MRA
MRA has high sensitivity and specificity for diagnosing aortic dissection. It can accurately assess the true and false lumens, the extent of the dissection, and the morphology of branch vessels. However, its longer scanning time makes it unsuitable for patients with claustrophobia or hemodynamic instability, limiting its use in the initial evaluation of acute aortic dissection.
Aortic DSA
DSA is now primarily used during endovascular repair procedures and is not routinely performed as a preoperative diagnostic method.
Diagnosis and Differential Diagnosis
For patients presenting with acute chest pain, it is important to evaluate their medical history, the nature of the chest pain, and physical signs. A scoring system is used based on three categories of high-risk factors (predisposing factors, pain characteristics, and physical signs). A score of 1 is assigned for each category if one or more features are present. Patients with scores in two or more categories are classified as high-risk, those with one category as intermediate-risk, and those with none as low-risk. For patients with suspected aortic dissection who are classified as intermediate- or high-risk, appropriate imaging studies should be arranged as soon as possible to confirm the diagnosis.
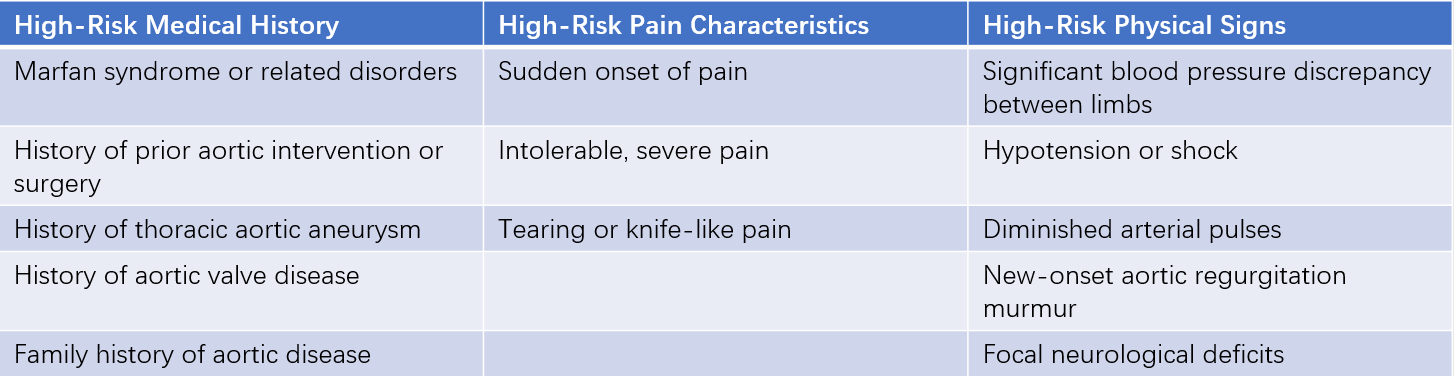
Table 1 High-risk features of aortic dissection
Since acute chest pain is the primary symptom in aortic dissection, the differential diagnosis primarily includes acute coronary syndrome and acute pulmonary embolism. Imaging studies are necessary to differentiate aortic dissection from intramural hematoma and penetrating aortic ulcer. Additionally, aortic dissection may cause compression of multiple vascular systems, leading to tissue ischemia or rupture into specific organs, requiring differentiation from related conditions.
Treatment
Once the diagnosis of aortic dissection is confirmed, a multidisciplinary team of aortic specialists should evaluate the patient’s condition, the extent of dissection, and the time since onset to develop the optimal treatment plan.
Immediate Management
Hemodynamic parameters, including blood pressure, heart rate, cardiac rhythm, and fluid balance, should be closely monitored. For patients with heart failure or hypotension, central venous pressure, pulmonary capillary wedge pressure, and cardiac output should also be monitored. Bed rest is recommended, along with effective pain relief and sedation. Intravenous morphine or hibernation therapy may be administered if necessary.
Subsequent Treatment Decisions
During the acute phase, all patients, regardless of whether surgical intervention is planned, should first receive intensive medical therapy.
For ascending aortic dissections, particularly those involving the aortic valve or with pericardial effusion, emergency surgical intervention is preferred.
For descending aortic dissections with rapid progression during the acute phase, a local vascular diameter ≥5 cm, or impaired organ perfusion, endovascular treatment (thoracic endovascular aortic repair, TEVAR) should be considered.
Medical Therapy
Beta-Blockers or Calcium Channel Blockers: These medications are used to lower blood pressure, reduce left ventricular wall tension, decrease myocardial contractility, and slow the heart rate to 60-80 beats per minute, preventing further extension of the dissection. For patients intolerant to beta-blockers, non-dihydropyridine calcium channel blockers can be used.
Intravenous sodium nitroprusside is the first-line choice to rapidly reduce systolic blood pressure to 100-120 mmHg or lower, preventing dissection progression. Other antihypertensive drugs, such as alpha-blockers, angiotensin-converting enzyme inhibitors, or diuretics, may be used as needed. Blood pressure should be maintained at the lowest level that ensures adequate perfusion of vital organs, avoiding symptoms of poor organ perfusion such as oliguria, myocardial ischemia, or neurological deficits.
Endovascular Treatment
Thoracic endovascular aortic repair (TEVAR) is an effective treatment for aortic dissection. A covered stent is implanted into the aorta to seal the proximal tear and expand the true lumen. For Stanford Type B aortic dissections with suitable anatomical conditions, TEVAR is often the preferred strategy. In recent years, advancements such as parallel stent techniques, branched stents, fenestration techniques, and customized stents based on 3D printing have been developed to effectively manage cases involving critical branch vessels.
Surgical Treatment
Open surgical repair remains the cornerstone of treatment for ascending aortic dissections. During surgery, the intimal tear can be repaired, the false lumen evacuated, and the aorta reconstructed. If the dissection involves the coronary arteries or aortic valve, these structures can be addressed simultaneously during the procedure.