Liver cirrhosis is the pathological stage of various chronic liver diseases characterized by chronic liver inflammation, diffuse fibrosis, pseudolobules, regenerative nodules, and intrahepatic and extrahepatic vascular proliferation. During the compensated phase, there are no obvious symptoms, while the decompensated phase is clinically marked by portal hypertension and liver function impairment. Patients often die from complications such as esophageal and gastric variceal bleeding, hepatic encephalopathy, infections, hepatorenal syndrome, portal vein thrombosis, and chronic multi-organ failure.
Etiology
There are more than ten causes of liver cirrhosis. In Western countries, alcohol and hepatitis C virus (HCV) are the most common etiologies.
Hepatitis viruses, fatty liver disease, immune disorders, and drugs or chemical toxins are frequent causes of cirrhosis. Other causes include:
Cholestasis
Any condition causing intrahepatic or extrahepatic bile duct obstruction and persistent cholestasis can lead to biliary cirrhosis. Depending on the cause of cholestasis, biliary cirrhosis can be classified as primary or secondary.
Circulatory Disorders
Obstruction of the hepatic vein and/or inferior vena cava (e.g., Budd-Chiari syndrome), chronic heart failure, and constrictive pericarditis (cardiac origin) can result in prolonged hepatic congestion, hepatocyte degeneration, and fibrosis, ultimately leading to cirrhosis.
Parasitic Infections
Schistosomiasis remains prevalent in some regions. Mature schistosome eggs are engulfed by hepatic macrophages, transforming into fibroblasts and forming fibrous nodules. Since the eggs primarily deposit near branches of the portal vein, fibrosis often causes portal perfusion disorders, with portal hypertension being a prominent feature of the resulting cirrhosis. Clonorchis sinensis, which parasitizes the intrahepatic and extrahepatic bile ducts, can cause bile duct obstruction and inflammation, eventually progressing to cirrhosis.
Genetic and Metabolic Disorders
Due to genetic or congenital enzyme deficiencies, certain metabolic products accumulate in the liver, causing hepatocyte necrosis and connective tissue proliferation. The main disorders include:
- Copper Metabolism Disorders: Also known as Wilson's disease or hepatolenticular degeneration, this autosomal recessive disorder of copper metabolism is caused by mutations in the ATP7B gene located on chromosome 13q14. The gene encodes the P1-type ATPase responsible for copper ion transport. Dysfunction of this enzyme leads to copper accumulation in the body, damaging the liver, brain, and other organs.
- Primary Hemochromatosis: Caused by genetic abnormalities on chromosome 6, this condition increases the absorption and transport of dietary iron by the intestinal mucosa, resulting in excessive iron deposition in the liver. This leads to fibrosis and organ dysfunction.
- α1-Antitrypsin Deficiency: α1-Antitrypsin (α1-AT) is a low-molecular-weight glycoprotein synthesized by the liver. Due to genetic defects, normal α1-AT is significantly reduced, while abnormal α1-AT, with a smaller molecular weight and lower solubility, accumulates extensively in hepatocytes, causing liver tissue damage and leading to cirrhosis.
Other conditions, such as galactosemia, hemophilia, type I tyrosinemia, and hereditary hemorrhagic telangiectasia, can also cause cirrhosis.
Unknown Causes
In some cases, the development of cirrhosis cannot be explained by currently recognized diseases and is termed cryptogenic cirrhosis. Before concluding that the cause of cirrhosis is unknown, careful evaluation of the aforementioned potential causes is necessary to avoid influencing the treatment of cirrhosis by addressing its etiology.
Pathogenesis and Pathology
Under the influence of various pathogenic factors, the liver undergoes chronic inflammation, a reduction in hepatocytes, diffuse fibrosis, and intrahepatic and extrahepatic vascular proliferation, eventually progressing to cirrhosis.
Hepatocytes can perish through the following mechanisms:
- Degeneration and necrosis;
- Degeneration and apoptosis;
- Gradual loss of epithelial characteristics, transforming into mesenchymal cells, a process known as epithelial-mesenchymal transition (EMT).
In healthy adults, the average lifespan of hepatocytes is 200–300 days, with slow renewal. However, after partial hepatectomy, the liver demonstrates strong regenerative capacity. Under conditions such as chronic inflammation or drug-induced damage, the ability of damaged hepatocytes in adults to regenerate becomes impaired.
Inflammatory and other pathogenic factors activate hepatic stellate cells, leading to their proliferation and migration, increased collagen synthesis, and decreased degradation. This collagen deposits in the space of Disse, causing the space to widen. Some hepatocytes, within the fibrotic microenvironment, may undergo EMT, further promoting the progression of liver fibrosis. Fibrous bands extend from the portal tracts and liver capsule toward the central veins of hepatic lobules. These fibrous septa encircle regenerative nodules or partition the remaining hepatic lobules, reconstructing them into pseudolobules, which are characteristic histopathological features of cirrhosis.
Alongside the development of liver fibrosis, there is significant vascular proliferation both within and outside the liver. Intrahepatic vascular proliferation leads to a reduction in fenestrations and a decrease in the number of hepatic sinusoidal endothelial cells. The gaps between these endothelial cells disappear, and a basement membrane forms, a process known as sinusoidal capillarization. This results in:
- Narrowing of hepatic sinusoids and obstruction of blood flow, impairing the transport of substances from the sinusoidal lumen to hepatocytes. This leads to hypoxia and nutritional deficiencies in hepatocytes, loss of microvilli on the hepatocyte surface, decreased hepatocyte function, degeneration, transformation into mesenchymal cells, increased apoptosis, and even cell death.
- Increased intrahepatic vascular resistance and elevated portal vein pressure. Positive feedback from vascular endothelial growth factor (VEGF) and platelet-derived growth factor B (PDGF-B) further promotes vascular proliferation within and outside the liver, perpetuating portal hypertension. The normal relationships among the portal vein, hepatic vein, and hepatic artery are disrupted, leading to the formation of collateral anastomoses.
Extrahepatic vascular proliferation increases the blood volume in the tributaries of the portal vein, exacerbating portal hypertension. This contributes to complications such as esophagogastric varices (EGV), splenomegaly, and portal hypertensive gastropathy.
Clinical Manifestations
Liver cirrhosis typically has an insidious onset and progresses slowly. Clinically, it is generally divided into the compensated and decompensated phases.
Compensated Phase
Most patients are asymptomatic or have mild symptoms, such as abdominal discomfort, fatigue, loss of appetite, indigestion, and diarrhea. These symptoms are often intermittent and may appear during physical exertion, mental stress, or concurrent illnesses. They tend to improve with rest or the use of digestive aids. The nutritional status of patients is generally adequate. Hepatomegaly depends on the type of cirrhosis, while splenomegaly is usually mild to moderate due to portal hypertension. Liver function tests are normal or show mild abnormalities.
Decompensated Phase
Symptoms become more pronounced, primarily involving liver function impairment and manifestations of portal hypertension.
Liver Function Impairment
Digestive and Absorptive Dysfunction
Symptoms include loss of appetite, nausea, anorexia, abdominal distension (worsening after meals), and diarrhea (especially after consuming fatty foods). These symptoms are often associated with gastrointestinal congestion and edema due to portal hypertension, impaired digestion and absorption, and intestinal dysbiosis.
Malnutrition and Sarcopenia
General health deteriorates, with weight loss, fatigue, and lethargy. In severe cases, patients may become bedridden due to weakness. The skin may appear dry or edematous.
Jaundice
Yellowing of the skin and sclera, dark urine, and progressive jaundice occur with extensive hepatocyte necrosis or liver failure. This is typically hepatocellular jaundice.
Bleeding and Anemia
Patients often experience nasal and gum bleeding, petechiae, ecchymosis, and gastrointestinal bleeding. These symptoms are related to reduced synthesis of clotting factors by the liver, hypersplenism, and increased capillary fragility.
Endocrine Disorders
The liver plays a critical role in the metabolism and degradation of various hormones. Hormones and their metabolites are actively involved in the pathogenesis and progression of liver diseases.
Sex Hormone Metabolism
Increased estrogen and decreased androgen levels are common. The former is related to reduced hepatic inactivation of estrogen, while the latter is associated with elevated estrogen levels suppressing pituitary gonadotropin release, leading to reduced androgen secretion by testicular Leydig cells. Male patients may experience decreased libido, testicular atrophy, hair loss, and gynecomastia. Female patients may present with menstrual irregularities, amenorrhea, and infertility. The appearance of spider angiomas and palmar erythema is linked to elevated estrogen levels.
Adrenal Cortical Function
Cholesterol, a precursor for adrenal corticosteroid synthesis, decreases in cirrhosis, leading to insufficient corticosteroid production. Elevated levels of endotoxins and pro-inflammatory factors suppress corticotropin-releasing factor, resulting in adrenal insufficiency. Increased melanocyte-stimulating hormone causes hyperpigmentation of the face and other exposed areas, leading to a dark, sallow complexion referred to as "liver face."
Antidiuretic Hormone (ADH)
Elevated ADH levels contribute to the formation of ascites.
Thyroid Hormones
Serum total T3 and free T3 levels decrease in cirrhotic patients, while free T4 levels are normal or slightly elevated. In severe cases, T4 levels may also decline. These changes correlate with the severity of liver disease.
Irregular Low-Grade Fever
Reduced hepatic inactivation of pyrogens and other factors, as well as secondary infections, may contribute to low-grade fever.
Hypoalbuminemia
Patients often exhibit lower limb edema and ascites due to decreased serum albumin levels.
Portal Hypertension
Portal hypertension is mostly intrahepatic in nature and often leads to complications such as esophagogastric variceal bleeding, ascites, splenomegaly, hypersplenism, hepatorenal syndrome, and hepatopulmonary syndrome. It represents a critical pathophysiological mechanism that exacerbates liver function decline following the primary etiology and is one of the leading causes of death in cirrhosis.
Formation of Portosystemic Collateral Circulation
Persistent portal hypertension promotes vascular proliferation within and outside the liver. Intrahepatic shunting occurs through connections between the portal vein and hepatic vein within fibrous septa, allowing portal blood to bypass hepatic lobules and flow into the hepatic vein through collateral vessels. Common extrahepatic portosystemic collateral circulations are described as follows:

Figure 1 Portosystemic collateral circulation in portal hypertension
1, Portal vein; 2, Splenic vein; 3, Coronary vein of the stomach; 4, Umbilical vein; 5, Esophagogastric varices (EGV); 6, Retzius veins; 7, Splenorenal shunt
Esophagogastric Varices (EGV)
The coronary vein of the stomach (a tributary of the portal vein system) forms an anastomosis with the esophageal and azygos veins (part of the systemic circulation) at the lower esophagus and gastric fundus, resulting in esophagogastric varices. Rupture and bleeding of these varices are the most common complications of portal hypertension in cirrhosis. Due to the thin walls and lack of elastic contractility of the varicose veins, bleeding is difficult to control and associated with high mortality.
Abdominal Wall Varices
Recanalization and proliferation of the umbilical vein and paraumbilical veins occur under portal hypertension. These veins drain into the superior and inferior vena cava, with superficial abdominal veins around the umbilicus radiating in a star-like pattern toward the upper and lower umbilical regions.
Hemorrhoidal Varices
The superior rectal vein, which drains into the inferior mesenteric vein and subsequently the portal vein, forms an anastomosis with the middle and inferior rectal veins (tributaries of the systemic circulation through the internal iliac vein) in the lower rectum. This results in hemorrhoidal varices. In some cases, patients discover cirrhosis due to rectal bleeding caused by hemorrhoids.
Retroperitoneal Collateral Varices
Numerous small branches, known as Retzius veins, connect the retroperitoneal portal vein to the inferior vena cava. Under portal hypertension, these veins become dilated and varicose, relieving portal pressure.
Splenorenal Shunts
Branches of the portal vein, such as the splenic and gastric veins, may communicate with the left renal vein, forming splenorenal shunts.
In addition to causing life-threatening events such as esophagogastric variceal bleeding (EGVB), the extensive abnormal shunting also impairs the liver's ability to uptake, metabolize, and degrade various substances, including the phagocytic and detoxifying functions of Kupffer cells. Toxins entering the portal vein from the intestines bypass the liver and directly enter the systemic circulation, leading to a series of pathophysiological changes such as hepatic encephalopathy, hepatorenal syndrome, spontaneous bacterial peritonitis, and prolonged drug half-life. Furthermore, the slow portal blood flow caused by these abnormal shunts is a contributing factor to portal vein thrombosis.
Splenomegaly and Hypersplenism
Splenomegaly is an early sign of portal hypertension in cirrhosis. Increased resistance to splenic vein outflow and retrograde transmission of portal pressure to the spleen result in passive congestive splenomegaly. This is accompanied by proliferation of splenic tissue and fibrosis. Additionally, antigens from the intestines enter the systemic circulation through portosystemic collateral vessels and are taken up by the spleen. Antigenic stimulation leads to proliferation of mononuclear phagocytes in the spleen, causing hypersplenism. Peripheral blood often shows varying degrees of thrombocytopenia, leukopenia, and anemia due to hyperplasia. This increases susceptibility to infections and bleeding. Splenomegaly is often more pronounced in schistosomal cirrhosis.
Ascites
Ascites is a result of both liver dysfunction and portal hypertension and represents a prominent clinical manifestation of the imbalance between portal and systemic circulations during the decompensated phase of cirrhosis. Patients often complain of abdominal distension. Large amounts of ascitic fluid can cause abdominal swelling resembling a frog's belly and may lead to umbilical hernias. The upward displacement of the diaphragm restricts its movement, causing dyspnea and palpitations.
The mechanisms of ascites formation involve the following:
- Portal Hypertension: Increased hydrostatic pressure in the visceral vascular beds of the abdominal cavity reduces reabsorption of interstitial fluid, leading to its leakage into the abdominal cavity. This is the decisive factor in ascites formation.
- Hypoalbuminemia: When albumin levels drop below 30 g/L, the colloid osmotic pressure of plasma decreases, causing fluid to leak into the abdominal cavity or interstitial spaces.
- Effective Circulating Blood Volume Deficiency: Reduced renal blood flow activates the renin-angiotensin system, leading to decreased glomerular filtration rates and reduced sodium and urine excretion, contributing to water and sodium retention.
- Impaired Hepatic Inactivation of Aldosterone and Antidiuretic Hormone: This results in secondary hyperaldosteronism and increased levels of antidiuretic hormone. Aldosterone acts on the distal renal tubules to enhance sodium reabsorption, while antidiuretic hormone acts on the collecting ducts to increase water reabsorption, leading to water and sodium retention and reduced urine output.
- Increased Hepatic Lymph Production: Elevated sinusoidal pressure increases hepatic lymph production, which exceeds the drainage capacity of the lymphatic system. Excess lymph leaks from the liver surface into the abdominal cavity, contributing to ascites formation.
Complications
Gastrointestinal Bleeding
Esophagogastric Variceal Bleeding (EGVB)
Portal hypertension is the primary cause of EGVB. Clinical manifestations include sudden massive hematemesis or melena, which can lead to hemorrhagic shock in severe cases.
Peptic Ulcer
Portal hypertension slows venous drainage of the gastric mucosa, impairing its barrier function and increasing the risk of gastric and duodenal ulcers, which may result in bleeding.
Portal Hypertensive Gastroenteropathy
Proliferation of portal tributary vessels, capillary dilation, and vessel wall defects lead to widespread oozing of blood. Portal hypertensive gastropathy often presents as recurrent or persistent mild hematemesis and melena, while portal hypertensive enteropathy typically manifests as recurrent melena or hematochezia.
Gallstone Disease
The prevalence is approximately 30%, with gallstones and extrahepatic bile duct stones being more common.
Infections
Patients with cirrhosis are prone to infections due to the following factors:
- Portal hypertension reduces the barrier function of the intestinal mucosa, increasing its permeability and allowing bacteria from the intestinal lumen to enter the bloodstream via the lymphatic system or portal vein.
- The liver is a vital immune organ, and cirrhosis severely impairs immune function.
- Hypersplenism or splenectomy results in reduced immune function.
- Cirrhosis is often accompanied by glucose metabolism disorders, and diabetes further reduces resistance to infections.
The site of infection varies depending on the patient’s underlying condition, with the following being common:
Spontaneous Bacterial Peritonitis (SBP)
SBP refers to acute bacterial peritonitis not caused by intra-abdominal organ infections. Ascitic fluid provides an excellent culture medium for bacteria, making patients with ascites prone to SBP. The causative organisms are predominantly Gram-negative bacilli.
Biliary Tract Infections
Biliary obstruction or partial obstruction caused by gallstones in the gallbladder or extrahepatic bile ducts is often accompanied by infection. Patients may experience abdominal pain and fever. Obstructive jaundice may occur if the common bile duct is obstructed, and hepatocellular jaundice may develop if infection further damages liver function.
Pulmonary, Intestinal, and Urinary Tract Infections
Gram-negative bacilli are the most common pathogens. However, due to the widespread use of broad-spectrum antibiotics and impaired immune function, infections caused by anaerobic bacteria and fungi are increasingly common.
Hepatic Encephalopathy (HE)
Hepatic encephalopathy refers to a syndrome of central nervous system dysfunction caused by metabolic disturbances due to hepatic insufficiency and/or portosystemic shunting in the context of cirrhosis. Approximately 50% of cirrhosis patients exhibit cerebral edema. In patients with a long disease course, the cerebral cortex becomes thinner, and the number of neurons and nerve fibers decreases. The pathogenesis involves the following mechanisms:
Ammonia Toxicity
Ammonia toxicity is a critical mechanism in the development of hepatic encephalopathy, particularly in portosystemic shunting-related hepatic encephalopathy. The digestive tract is the primary site of ammonia production, where ammonia exists in two forms: non-ionized ammonia (NH3) and ionized ammonia (NH4+). When the colonic pH exceeds 6, NH4+ is converted to NH3, which readily diffuses into the bloodstream through the intestinal mucosa. When the pH is below 6, NH3 moves from the blood into the intestinal lumen and is excreted with feces. In liver failure, the liver’s ability to metabolize NH3 from the portal vein is significantly reduced, leading to elevated systemic NH3 levels. In the presence of portosystemic shunting, intestinal NH3 bypasses the liver and directly enters the systemic circulation, further increasing blood NH3 levels. Elevated systemic NH3 crosses the blood-brain barrier and interferes with brain function in several ways:
- Interfering with the tricarboxylic acid cycle in brain cells, leading to energy deficiency.
- Increasing the uptake of tyrosine, phenylalanine, and tryptophan by the brain, which have inhibitory effects on brain function.
- Elevating brain NH3 levels, which enhances glutamine synthesis, causing neuronal swelling and cerebral edema.
- Directly disrupting neuronal electrical activity.
- Upregulating benzodiazepine receptor expression in astrocytes, promoting chloride ion influx and inhibiting neuronal transmission.
False Neurotransmitters
Cirrhosis impairs the liver’s ability to clear intestinally derived tyramine and phenylethylamine. These amines enter the brain and are converted to β-hydroxytyramine and phenylethanolamine, respectively. Due to their chemical similarity to normal neurotransmitters like norepinephrine but weak or absent neurotransmitter activity, they are referred to as false neurotransmitters. False neurotransmitters disrupt neuronal signal transmission.
Tryptophan
Tryptophan in the bloodstream is usually bound to albumin and cannot easily cross the blood-brain barrier. In liver disease, reduced albumin synthesis increases free tryptophan levels. Once crossing the blood-brain barrier, tryptophan is metabolized into the inhibitory neurotransmitter serotonin (5-HT) and 5-hydroxyindoleacetic acid, contributing to hepatic encephalopathy. This is particularly associated with early symptoms such as altered sleep patterns and disrupted circadian rhythms.
Manganese Ions
Manganese, which is secreted into the bile by the liver, has neurotoxic effects. Under normal conditions, manganese is excreted through the intestines. In liver disease, impaired biliary excretion allows manganese to enter the brain via the bloodstream, contributing to hepatic encephalopathy.
Common precipitating factors include gastrointestinal bleeding, excessive potassium-wasting diuretics, paracentesis for ascites, high-protein diets, sedatives, anesthetics, constipation, uremia, surgical procedures, and infections.
Hepatic encephalopathy lacks distinctive features compared to other metabolic encephalopathies. Clinical manifestations include dysfunction of higher central nervous system functions, abnormal motor activity, and altered reflexes. The clinical course is divided into five stages.

Table 1 Clinical staging of hepatic encephalopathy
Portal Vein Thrombosis or Cavernous Transformation
Portal vein thrombosis occurs due to blood flow stasis in the portal vein, affecting the main trunk of the portal vein, the superior mesenteric vein, the inferior mesenteric vein, or the splenic vein. This reduces hepatic blood supply and accelerates liver failure. Initially intrahepatic portal hypertension may extend to prehepatic portal hypertension. When the thrombosis spreads to the superior mesenteric vein, significant intestinal congestion occurs, progressively impairing small intestinal function. This complication is relatively common, especially after splenectomy, where the incidence of portal vein or splenic vein thrombosis can exceed 55%.
The clinical manifestations of portal vein thrombosis vary widely. When thrombosis develops slowly and is confined to the left or right branches of the portal vein or the extrahepatic portal vein, the development of collateral circulation often prevents significant symptoms, and the condition may go undetected until identified by imaging studies. Severe portal vein thrombosis, which significantly obstructs hepatic blood flow, can result in refractory esophagogastric variceal bleeding (EGVB), moderate to severe abdominal distension and pain, refractory ascites, intestinal necrosis, and hepatic encephalopathy. Paracentesis may reveal hemorrhagic ascitic fluid.
Cavernous transformation of the portal vein (CTPV) refers to chronic partial or complete obstruction of the portal vein trunk or its intrahepatic branches, leading to narrowing, atrophy, or even disappearance of the portal vein trunk. In this condition, a network of small, tortuous collateral vessels forms around the portal vein. CTPV is associated with splenectomy, endoscopic variceal ligation (EVL), portal vein inflammation, portal vein thrombosis, polycythemia, and tumor invasion.
Electrolyte and Acid-Base Imbalances
Electrolyte imbalances are commonly caused by prolonged insufficient sodium intake, diuretic use, large-volume paracentesis for ascites, diarrhea, and secondary hyperaldosteronism. Hypokalemia, hypochloremia, and metabolic alkalosis are known to trigger hepatic encephalopathy (HE). Persistent severe hyponatremia (serum sodium <125 mmol/L) often leads to hepatorenal syndrome and is associated with a poor prognosis.
Hepatorenal Syndrome (HRS)
Hepatorenal syndrome is characterized by renal failure without intrinsic kidney disease. It results from severe portal hypertension and hyperdynamic circulation in the viscera, which significantly reduces systemic blood flow. Various vasodilatory substances such as prostaglandins, nitric oxide, glucagon, atrial natriuretic peptide, endotoxins, and calcitonin gene-related peptide are not adequately inactivated by the liver, causing systemic vasodilation and cardiac dysfunction. As a result, renal vasoconstriction occurs, leading to reduced renal blood flow, especially in the renal cortex, and subsequent renal failure.
Clinically, HRS presents with oliguria, anuria, and azotemia. In 80% of patients with the acute form, death occurs within approximately two weeks. The chronic form is more common and is often associated with refractory ascites. Renal failure progresses slowly and may remain stable for several months, but acute triggers can cause rapid progression to the acute form, resulting in death.
Hepatopulmonary Syndrome (HPS)
Hepatopulmonary syndrome occurs in the context of cirrhosis, excluding primary cardiopulmonary diseases. It is characterized by symptoms of respiratory distress and hypoxemia, such as cyanosis and clubbing of the fingers and toes. These symptoms are related to pulmonary vascular dilation and impaired arterial oxygenation. The prognosis is poor.
Primary Liver Cancer
Details on this topic can be found in the relevant section.
Diagnosis
The diagnostic process involves determining the presence of cirrhosis, identifying its etiology, assessing liver function, staging the disease, and diagnosing complications.
Determining the Presence of Cirrhosis
Imaging studies may reveal wavy liver contours, atrophy of certain liver lobes, and nodular changes. Ultrasound findings such as enhanced and heterogeneous liver echogenicity and nodular alterations support the diagnosis of cirrhosis. Clinical diagnosis of decompensated cirrhosis is typically based on the simultaneous presence of evidence of liver dysfunction and portal hypertension. When evidence of liver dysfunction and portal hypertension is insufficient, and imaging findings are inconclusive, a histopathological examination showing pseudolobule formation can establish the diagnosis.
Liver Dysfunction
This includes the aforementioned clinical manifestations and laboratory tests reflecting hepatocyte injury, impaired bilirubin metabolism, and reduced synthetic function of the liver.
Portal Hypertension
The formation of portosystemic collateral circulation, splenomegaly, and ascites are key indicators of portal hypertension.
Physical examination findings such as abdominal wall varices and esophagogastric varices observed during endoscopy reflect the development of portosystemic collateral circulation. Ultrasound may detect a portal vein trunk diameter >13 mm or a splenic vein diameter >8 mm, as well as the velocity and direction of portal vein blood flow. Abdominal contrast-enhanced CT and portal vein imaging can clearly and accurately display various changes in portal vein tributaries, portal vein thrombosis, cavernous transformation, and arteriovenous fistulas, providing a comprehensive assessment of portal hypertension.
Splenomegaly, mild ascites, and changes in liver morphology can be confirmed using ultrasound, CT, or MRI, which are more sensitive and accurate than physical examination. A reduced platelet count is an early indicator of portal hypertension. As splenomegaly and hypersplenism worsen, red and white blood cell counts also decrease.
Ascites in cirrhosis without infection is typically transudative. When spontaneous bacterial peritonitis (SBP) is present, the ascitic fluid may show typical exudative characteristics or features intermediate between transudate and exudate. A serum-ascites albumin gradient (SAAG) ≥ 11 g/L strongly suggests portal hypertension as the cause of ascites, while a SAAG < 11 g/L is more indicative of non-portal hypertension-related causes, such as tuberculosis or malignancy.
Identifying the Cause of Cirrhosis
When diagnosing cirrhosis, efforts should be made to identify the underlying cause to facilitate targeted treatment.
Liver Function Assessment
Details can be found in the relevant section.
Staging of Cirrhosis
Cirrhosis has been classified into two stages: the compensated stage, where no complications are present, and the decompensated stage, characterized by severe complications such as esophagogastric variceal bleeding (EGVB), ascites, infections, or hepatic encephalopathy (HE).
In recent years, based on extensive clinical data on the natural history of cirrhosis, a four-stage clinical classification has been proposed. Upon entering the decompensated stage, the median survival time decreases from over 12 years in the compensated stage to approximately 2–4 years.
Some patients with decompensated cirrhosis may achieve a state of "recompensation" after effective control of the underlying cause, reduction of portal hypertension, and improvement of the hepatic immune microenvironment. This state is defined as being stable for at least 12 months and includes the following criteria:
- Resolution of ascites without reliance on diuretics.
- Absence of recurrent EGVB.
- Remission of hepatic encephalopathy without reliance on lactulose or rifaximin.
- Sustained stability of albumin and bilirubin levels.
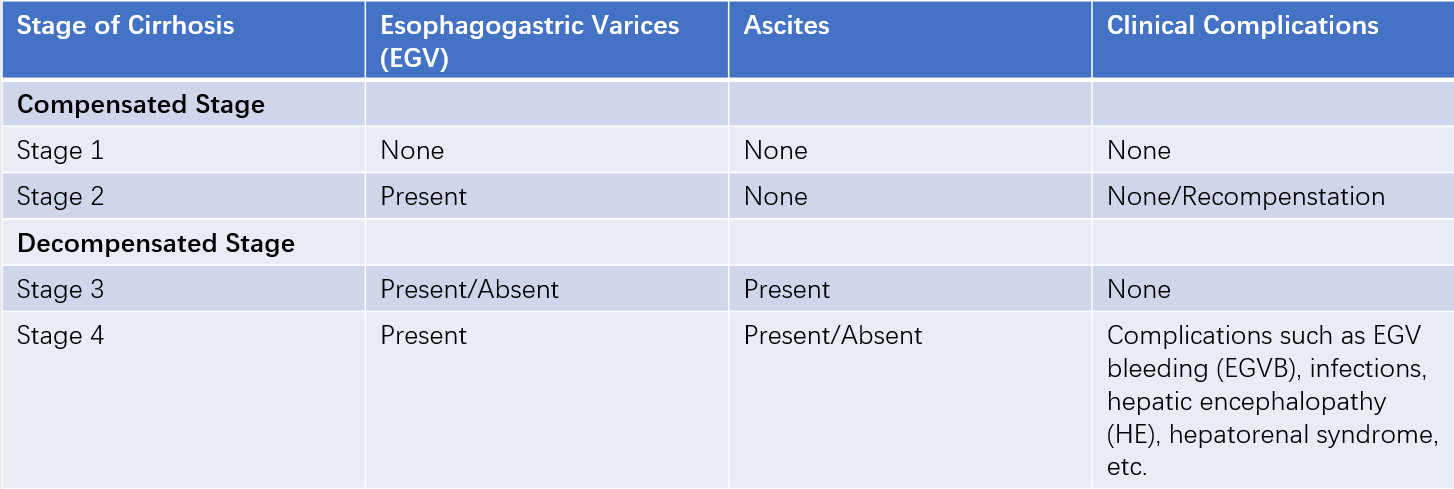
Table 2 Staging of cirrhosis
Diagnosis of Complications
EGVB and Portal Hypertensive Gastroenteropathy
Endoscopy, contrast-enhanced abdominal CT, and portal vein imaging are important diagnostic tools.
Cholelithiasis
Abdominal ultrasound and magnetic resonance cholangiopancreatography (MRCP) are commonly used.
Spontaneous Bacterial Peritonitis (SBP)
Patients with a slow onset may present with low-grade fever, abdominal distension, or persistent ascites. Rapid disease progression may result in significant abdominal pain, rapid accumulation of ascites, and severe cases may develop hepatic encephalopathy or toxic shock. Physical examination often reveals varying degrees of diffuse abdominal tenderness and peritoneal irritation. The ascitic fluid appears turbid, with biochemical and microscopic findings suggesting an exudative nature, and pathogenic bacteria may be cultured from the fluid.
Hepatic Encephalopathy (HE)
The primary diagnostic criteria include:
- A history of severe liver disease and/or extensive portosystemic shunting, along with precipitating factors for hepatic encephalopathy.
- Clinical manifestations consistent with hepatic encephalopathy.
- Significant abnormalities in liver function tests and/or elevated blood ammonia levels.
- Exclusion of cerebrovascular accidents, intracranial tumors, and other conditions through head CT or MRI.
A small subset of hepatic encephalopathy patients may lack a clear history of liver disease and present predominantly with psychiatric symptoms, increasing the risk of misdiagnosis. Therefore, for patients with psychiatric symptoms, assessing their liver disease history and performing liver function tests should be routine to exclude hepatic encephalopathy.
Portal Vein Thrombosis or Cavernous Transformation
When clinically suspected, contrast-enhanced abdominal CT and portal vein imaging can confirm the diagnosis.
Hepatorenal Syndrome (HRS)
The diagnosis of hepatorenal syndrome requires the following criteria:
- Cirrhosis with ascites.
- For acute (Type I) HRS, serum creatinine doubles from baseline within two weeks or exceeds 226 μmol/L (25 mg/L). For chronic (Type II) HRS, serum creatinine exceeds 133 μmol/L (15 mg/L).
- No improvement in serum creatinine (>133 μmol/L) after discontinuing diuretics for more than two days and administering albumin [1 g/(kg·day), maximum dose 100 g/day].
- Exclusion of shock.
- No recent use of nephrotoxic drugs or vasodilators.
- Exclusion of intrinsic kidney disease, such as proteinuria >500 mg/day, >50 red blood cells per high-power field on microscopy, or evidence of renal parenchymal disease on imaging.
Hepatopulmonary Syndrome (HPS)
Hepatopulmonary syndrome is characterized by clubbing of the fingers, cyanosis, and severe hypoxemia (PaO2 < 70 mmHg) in cirrhotic patients. Diagnostic tools include 99mTc-macroaggregated albumin (MAA) scanning and contrast-enhanced two-dimensional echocardiography, which can demonstrate pulmonary capillary dilation.
Differential Diagnosis
Diseases Causing Ascites and Abdominal Distension
These include tuberculous peritonitis, intra-abdominal malignancies, nephrotic syndrome, constrictive pericarditis, and large ovarian cysts.
Hepatomegaly and Nodular Lesions in the Liver
These should be distinguished from chronic hepatitis, hematologic diseases, primary liver cancer, and schistosomiasis.
Complications of Cirrhosis
Upper gastrointestinal bleeding should be differentiated from peptic ulcers, erosive hemorrhagic gastritis, and gastric cancer.
Hepatic encephalopathy should be differentiated from hypoglycemia, diabetic ketoacidosis, uremia, cerebrovascular accidents, central nervous system infections, and sedative overdose.
Hepatorenal syndrome should be differentiated from chronic glomerulonephritis and acute tubular necrosis.
Hepatopulmonary syndrome should be distinguished from pulmonary infections, asthma, and other conditions.
Treatment
For patients in the compensated stage, the goal of treatment is to delay liver function decompensation, prevent hepatocellular carcinoma, and attempt to reverse the pathological changes. For patients in the decompensated stage, the focus is on improving liver function, treating complications, and delaying or reducing the need for liver transplantation.
Protecting or Improving Liver Function
Addressing or Reducing the Underlying Cause
Treatment includes antiviral therapy for hepatitis and addressing other underlying causes.
Avoiding Hepatotoxic Drugs
Unnecessary or ineffective medications should be avoided to reduce the metabolic burden on the liver.
Maintaining Enteral Nutrition
In cirrhosis, insufficient carbohydrate intake can lead to the body utilizing protein for energy, further increasing the liver's metabolic burden. Enteral nutrition is the best way for the body to obtain energy and plays a critical role in maintaining liver function and preventing gut-derived infections. As long as the gastrointestinal tract is functional, enteral nutrition should be encouraged to reduce reliance on parenteral nutrition.
Cirrhosis is often accompanied by indigestion, so easily digestible foods should be consumed, with carbohydrates as the primary energy source. Protein intake should be adjusted to the patient's tolerance, supplemented with multivitamins. Pancreatic enzymes may be provided to aid digestion. For patients with reduced appetite or food intolerance, pre-digested enteral nutrition formulas containing protein hydrolyzed into small peptides can be used. In cases of liver failure or impending hepatic encephalopathy, protein intake should be reduced.
Protecting Hepatocytes
For bile stasis, minimally invasive surgery to relieve biliary obstruction can prevent further liver damage. Ursodeoxycholic acid can be administered to reduce the proportion of hydrophobic bile acids (e.g., chenodeoxycholic acid) in bile, which can otherwise damage hepatocyte membranes. Other hepatoprotective agents, such as ademetionine, may also be used.
While certain hepatoprotective drugs, such as polyene phosphatidylcholine, silymarin, reduced glutathione, and diammonium glycyrrhizinate, have pharmacological evidence, robust clinical evidence is generally lacking. It is recommended to limit the use of these agents to no more than two simultaneously.
Treatment of Portal Hypertension and Its Complications
Ascites
Restricting Sodium and Water Intake
Sodium intake should be limited to less than 2.0 g/day, and fluid intake should be restricted to less than 1,000 ml/day. In cases of hyponatremia, fluid intake should be further restricted to less than 500 ml/day.
Diuretics
A combination of potassium-sparing and potassium-wasting diuretics, such as spironolactone and furosemide, is commonly used at a ratio of approximately 100:40. Treatment typically begins with spironolactone 60 mg/day combined with furosemide 20 mg/day, gradually increasing to spironolactone 100 mg/day and furosemide 40 mg/day. If diuresis is insufficient, intravenous albumin may be administered as needed.
Diuresis should not proceed too rapidly, as this may trigger hepatic encephalopathy or hepatorenal syndrome. Refractory ascites is defined as ascites that does not respond to sodium restriction and high-dose diuretics or rapidly recurs after therapeutic paracentesis.
Transjugular Intrahepatic Portosystemic Shunt (TIPS)
TIPS involves creating an intrahepatic shunt between the hepatic vein and the portal vein via an interventional approach to reduce portal pressure. This can alleviate portal hypertension-related ascites and esophagogastric variceal bleeding (EGVB). Compared to other treatments for portal hypertension, TIPS effectively reduces portal pressure, improves renal perfusion, and significantly reduces or eliminates ascites.
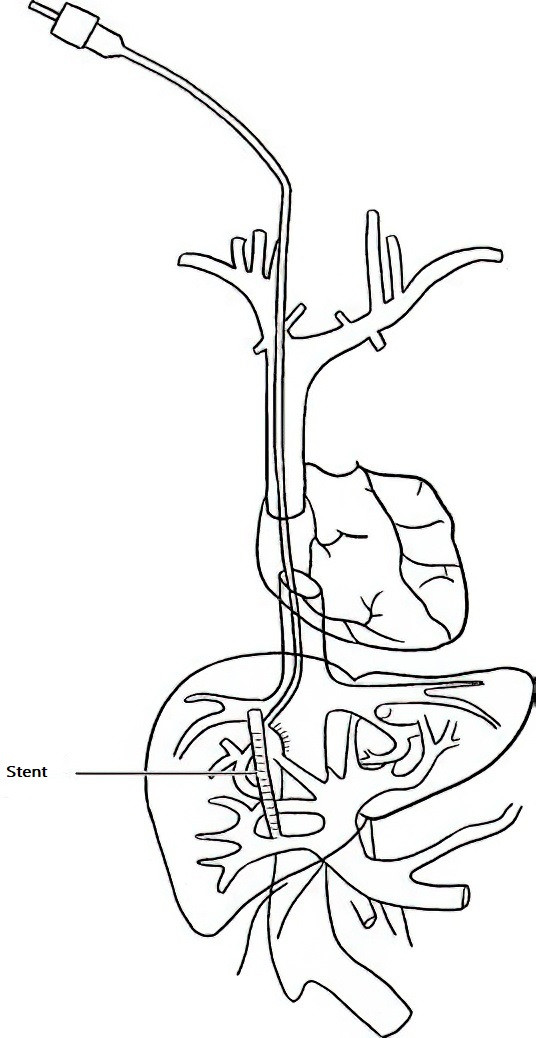
Figure 2 Transjugular intrahepatic portosystemic shunt (TIPS)
For patients whose liver function can be stabilized or improved through appropriate treatment, TIPS can maintain its effect for a relatively long period. Most patients undergoing TIPS do not require long-term sodium restriction, fluid restriction, or diuretics, reducing the need for liver transplantation. However, TIPS is not recommended for patients with severe right heart failure, congestive heart failure (ejection fraction <40%), severe valvular dysfunction, severe pulmonary hypertension (mean pulmonary artery pressure >45 mmHg), or Grade III or higher hepatic encephalopathy.
Paracentesis with Albumin Infusion
For patients with refractory ascites who are not suitable for TIPS, have contraindications to TIPS, or have missed the opportunity for TIPS, palliative treatment may involve therapeutic paracentesis with albumin infusion. Typically, 8 g of albumin is infused for every 1,000 ml of ascitic fluid removed. However, this method provides only short-term symptom relief and may lead to complications such as hepatorenal syndrome or hepatic encephalopathy.
Spontaneous Bacterial Peritonitis (SBP)
Antibiotics with low hepatotoxicity that target primarily Gram-negative bacilli and also Gram-positive cocci, such as cefoperazone or quinolones, are often used. If the treatment response is unsatisfactory, adjustments should be made based on clinical response and drug susceptibility results.
Since SBP is prone to recurrence, the duration of antibiotic therapy should not be less than two weeks. As SBP often originates from gut-derived infections, maintaining bowel regularity and preserving intestinal flora are important. Ascitic fluid serves as a favorable medium for bacterial growth, so controlling ascites is also a key part of managing this complication.
Treatment and Prevention of Esophagogastric Variceal Bleeding (EGVB)
General Emergency Measures and Volume Resuscitation
Details on general emergency measures and volume resuscitation can be found in the section on gastrointestinal bleeding. Blood volume replacement should not aim for full restoration but should suffice to maintain basic tissue perfusion and circulatory stability. Emergency surgical interventions are associated with high complication and mortality rates and are rarely used.
Hemostatic Measures
Medications
Early administration of visceral vasoconstrictors, such as vasopressin and its analogs (e.g., pituitrin, terlipressin), as well as somatostatin and its analogs (e.g., octreotide), is used to reduce portal venous blood flow and lower portal pressure to achieve hemostasis. Somatostatin and octreotide are the most commonly used drugs for EGVB due to their minimal impact on systemic hemodynamics and fewer adverse effects.
Somatostatin is administered as an initial intravenous bolus of 250 μg, followed by continuous intravenous infusion at 250 μg/h. Due to its short half-life, infusion must not be interrupted. If interrupted for more than 5 minutes, the initial dose should be re-administered. Octreotide, a somatostatin analog with a longer half-life, is given as an initial intravenous bolus of 100 μg, followed by continuous infusion at 25–50 μg/h.
Terlipressin is initially administered at 2 mg every 4 hours, and once bleeding is controlled, the dose can be reduced to 1 mg twice daily for a total of 5 days.
For patients with advanced cirrhosis, third-generation cephalosporins can be used, which not only aid in hemostasis but also reduce the risk of post-hemostasis infections.
Endoscopic Therapy
During active bleeding, emergency endoscopic variceal ligation (EVL) can be performed. This is a local devascularization procedure in which esophageal varices are ligated with rubber bands via an endoscope. The varices undergo ischemic necrosis, granulation tissue proliferation, and scar formation, leading to closure of the varices.
EVL does not reduce portal hypertension and is suitable for isolated esophageal varices without gastric varices. For gastric or ectopic variceal bleeding, tissue glue injection can be used.
Vascular Interventional Therapy
TIPS achieves a hemostasis rate of up to 95% for acute massive bleeding. For patients with massive bleeding or those with a low likelihood of successful endoscopic treatment, TIPS should be performed within 72 hours (preferably within 24 hours). Elective TIPS is typically reserved for patients with Child-Pugh scores below B. However, in cases of acute massive EGVB, TIPS can be performed in patients with Child-Pugh scores up to C14. The advantages of TIPS include minimal invasiveness, rapid recovery, fewer complications, and reliable efficacy.
Balloon-occluded retrograde transvenous obliteration (BRTO) is suitable for gastric varices with gastrorenal shunting.
Balloon Tamponade
Balloon tamponade is used temporarily in cases of massive bleeding where pharmacological therapy fails and endoscopic or TIPS procedures are unavailable, serving as a "bridge" to more definitive hemostatic measures. A Sengstaken-Blakemore tube is inserted through the nasal passage, with the gastric balloon inflated (pressure 50–70 mmHg) and traction applied to compress the gastric fundus. If bleeding persists, the esophageal balloon can be inflated (pressure 35–45 mmHg) to compress esophageal varices.
To prevent mucosal erosion, the duration of balloon compression should generally not exceed 24 hours. After deflation, compression can be reapplied if necessary. While balloon tamponade provides effective short-term hemostasis, it is associated with significant discomfort and complications, and its use is not recommended for long durations. The risk of early rebleeding after discontinuation is high.
Primary Prevention
Primary prevention is aimed at patients with esophagogastric varices who have not yet experienced bleeding and includes:
- Treatment of the Underlying Cause.
- Nonselective Beta-Blockers: These reduce visceral hyperdynamic circulation by constricting visceral blood vessels. Commonly used agents include propranolol and carvedilol. The therapeutic dose should maintain a heart rate of no less than 55 beats per minute and systolic blood pressure of no less than 90 mmHg. These drugs are not suitable for patients with refractory ascites.
- Endoscopic Variceal Ligation (EVL): This can be used for moderate esophageal varices.
Secondary Prevention
Secondary prevention targets patients who have already experienced EGVB to prevent rebleeding. The rebleeding rate after the first episode of bleeding can reach 60%, with a mortality rate of 33%. Therefore, secondary prevention of EGVB should be emphasized and initiated as early as the 6th day after bleeding.
Patients Who Underwent TIPS During Acute Bleeding
These patients may not require additional medication to prevent variceal bleeding but should undergo Doppler ultrasound every 3–6 months to monitor shunt patency.
Patients Who Did Not Undergo TIPS During Acute Bleeding
Rebleeding prevention methods include:
- Partial portosystemic shunting, such as TIPS.
- Devascularization procedures, including EVL or the injection of liquid embolic agents or other embolic materials into esophagogastric varices via endoscopic or vascular interventional approaches.
- Medications similar to those used for primary prevention.
The choice of these methods theoretically depends on the pathophysiology of portal hypertension, and specific treatment strategies should be based on findings from contrast-enhanced abdominal CT and portal venography. These imaging studies help determine the status of portosystemic collateral circulation, the degree of esophagogastric varices, the presence of portal vein thrombosis, cavernous transformation, or arteriovenous fistulas. Treatment selection should also consider liver function classification, contraindications, and patient preferences.
Hepatic Encephalopathy (HE)
Management focuses on eliminating precipitating factors, maintaining liver function, promoting ammonia metabolism and clearance, and regulating neurotransmitters.
Early Identification and Removal of Precipitating Factors
Correction of Electrolyte and Acid-Base Imbalances
Hypokalemic alkalosis is a common internal environment disturbance in cirrhotic patients, often resulting from reduced food intake, excessive diuresis, or large-volume paracentesis of ascitic fluid. Nutritional support should be emphasized, and diuretic doses should be carefully controlled to avoid excessive use.
Prevention and Control of Infections
Improvement of Gut Microbiota and Reduction of Nitrogenous Toxins in the Gut
Control of Bleeding and Removal of Intestinal Blood Accumulation
Upper gastrointestinal bleeding is a significant precipitating factor for HE. After achieving hemostasis, intestinal blood can be cleared using oral lactulose for laxative effects or through enemas with saline or weak acid solutions (e.g., dilute acetic acid).
Management of Constipation
Lactulose can be administered to ensure 1–2 soft bowel movements per day. Lactulose, a synthetic disaccharide, is not digested in the small intestine and is broken down in the colon by bacteria into lactic acid and acetic acid, which lower the intestinal pH. This acidic environment promotes the growth of non-urease-producing lactobacilli, reducing ammonia production by gut bacteria. Additionally, the acidic environment decreases ammonia absorption and facilitates the diffusion of ammonia from the blood into the gut for excretion. Lactulose is effective in all stages of HE, including minimal HE.
Oral Antibiotics
Antibiotics can inhibit urease-producing bacteria in the gut, reducing ammonia production. Commonly used antibiotics include rifaximin, metronidazole, and levofloxacin. Rifaximin is a broad-spectrum, highly effective antibiotic that inhibits gut bacteria, is not absorbed systemically, and acts locally in the gastrointestinal tract. The recommended dose is 0.8–1.2 g/day, divided into 2–3 doses. In patients with cirrhosis, where the gut mucosal barrier is weakened, orally absorbable antibiotics like levofloxacin can reduce bacterial load in the gut lumen and help eliminate bacteria that enter the portal circulation.
Cautious Use of Sedatives and Hepatotoxic Drugs
Sedatives, hypnotics, analgesics, and anesthetics can precipitate HE and should be avoided, especially in patients with cirrhosis and severe hepatic dysfunction. In cases of agitation or seizures, opioids, barbiturates, and benzodiazepines should not be used. Antihistamines like promethazine or chlorpheniramine may be considered as alternatives.
Nutritional Support
Adequate caloric intake should be ensured to avoid hypoglycemia, and various vitamins should be supplemented. Plasma or albumin may be administered as needed. During the acute phase of HE, protein intake should be restricted for several days (limited to 20 g/day in stages 1–2 HE). Once the patient regains consciousness, protein intake can be gradually increased from 20 g/day to 1 g/kg/day. For patients with protein intolerance after portosystemic shunting, large amounts of dietary protein should be avoided, but small amounts of continuous protein supplementation should still be maintained.
Promotion of Ammonia Metabolism
L-ornithine-L-aspartate is commonly used. Ornithine increases the activity of carbamoyl phosphate synthetase and ornithine carbamoyltransferase, and it also participates in the urea cycle to reduce blood ammonia levels. Aspartate promotes the activity of glutamine synthetase, facilitating the utilization and consumption of ammonia in the brain and kidneys to synthesize glutamate and glutamine, thereby lowering blood ammonia levels and alleviating cerebral edema. While other agents like sodium or potassium glutamate and arginine theoretically reduce blood ammonia levels and are widely used in clinical practice, evidence supporting their efficacy remains inconclusive.
Regulation of Neurotransmitters
Flumazenil
Flumazenil antagonizes the neuroinhibitory effects of endogenous benzodiazepines and has an arousal effect in some patients with stage 3–4 HE. Intravenous administration of 0.5–1 mg flumazenil can take effect within minutes, although the duration of action is typically short, lasting less than 4 hours.
Reduction or Antagonism of False Neurotransmitters
Branched-chain amino acid (BCAA) formulations, which primarily consist of leucine, isoleucine, and valine, competitively inhibit the entry of aromatic amino acids into the brain, reducing the formation of false neurotransmitters. Although the efficacy of BCAA supplementation remains controversial, it can help improve nitrogen balance in malnourished patients with protein intolerance.
Blockage of Portosystemic Shunting
HE caused by TIPS is often transient and self-limiting as liver function improves, urine output increases, and intestinal congestion decreases post-procedure. In rare cases, interventional procedures to reduce the shunt diameter may be required. For severe portosystemic collateral circulation resulting from cirrhotic portal hypertension, abnormal shunting can be addressed through a combination of TIPS and interventional variceal embolization.
Treatment of Other Complications
Cholelithiasis
Conservative medical management is preferred. Surgical intervention should be avoided as much as possible, especially in patients with Child-Pugh C liver function, since the surgical mortality rate for cirrhosis complicated by cholelithiasis is approximately 10%.
Infections
For infections associated with cirrhosis, empirical antibiotic therapy should be initiated immediately upon suspicion. Antibiotic selection for spontaneous bacterial peritonitis, biliary tract infections, and intestinal infections should prioritize broad-spectrum agents with adequate dosing and minimal hepatic or renal toxicity. Third-generation cephalosporins, such as cefoperazone combined with sulbactam, are the first choice. Other options include fluoroquinolones, piperacillin-tazobactam, and carbapenems, which can be used based on the patient's condition. Once pathogenic bacteria are identified, narrow-spectrum antibiotics should be selected based on susceptibility testing.
Portal Vein Thrombosis (PVT)
For acute symptomatic thrombosis, surgical intervention should be evaluated if signs of intestinal ischemia or necrosis arise. For non-acute symptomatic thrombosis, treatment decisions should be based on the severity, extent, and progression of the thrombosis. Anticoagulant therapy may be considered and can include vitamin K antagonists (e.g., warfarin), heparins (e.g., low-molecular-weight heparin), or novel oral anticoagulants (e.g., rivaroxaban, dabigatran). TIPS is indicated for patients with cirrhosis who fail anticoagulation therapy, have contraindications to anticoagulation, or present with severe complications such as esophagogastric variceal bleeding or refractory ascites.
Hyponatremia in Cirrhosis
Mild cases may improve with water restriction. For moderate to severe cases, vasopressin V2 receptor antagonists (e.g., tolvaptan) can be used to enhance renal water excretion, reduce water reabsorption, and increase serum sodium levels.
Hepatorenal Syndrome (HRS)
TIPS can help prevent the progression of slow-onset HRS to acute-onset HRS. Liver transplantation remains the most effective treatment for both types of HRS. While awaiting transplantation, measures to protect renal function include intravenous albumin supplementation, the use of vasopressin analogs, TIPS, hemodialysis, and artificial liver support systems.
Hepatopulmonary Syndrome (HPS)
Oxygen therapy and hyperbaric oxygen chambers are suitable for mild or early-stage cases, as they increase alveolar oxygen concentration and pressure, facilitating oxygen diffusion. Liver transplantation can reverse pulmonary vasodilation and significantly improve oxygen partial pressure, oxygen saturation, and pulmonary vascular resistance.
Hypersplenism
Partial splenic artery embolization and TIPS are the main treatments. Conventional total splenectomy is no longer recommended due to the high risk of postoperative portal vein thrombosis and severe infections.
Surgical Interventions
Shunting, devascularization, and flow-restricting surgeries for portal hypertension are now less commonly used due to advancements in endoscopic and interventional minimally invasive techniques. TIPS, with its advantages of being minimally invasive, precise, repeatable, and effective, has evolved from a bridging therapy before liver transplantation into a method that can significantly extend survival when combined with meticulous medical management. Liver transplantation remains the best treatment option for end-stage cirrhosis. Proper timing of surgery and thorough preoperative preparation can improve surgical survival rates.
Patient Education
Rest
Patients should avoid strenuous physical activity and high-intensity exercise. Those in the compensated stage may engage in light physical labor, while those in the decompensated stage should rest in bed more frequently. Maintaining emotional stability and reducing psychological stress is important.
Alcohol and Medication
Strict abstinence from alcohol is necessary. Unnecessary or unproven medications, over-the-counter analgesic and anti-inflammatory combination drugs, unregulated remedies, and health supplements should be avoided to reduce the metabolic burden on the liver and prevent hepatotoxic damage. For patients with insomnia, sedative and hypnotic drugs should be used cautiously under medical supervision.
Dietary Considerations
For patients with esophagogastric varices, food should not be consumed too quickly or in large quantities. Spicy, rough-textured foods should be avoided, and care should be taken to avoid swallowing bones or sharp objects when eating meat with bones. The diet should primarily consist of easily digestible, low-gas-producing grains, supplemented with small amounts of protein and fat. Vegetables and fruits should be consumed regularly, and seasoning should be mild. Bowel movements should be kept regular without straining. Common triggers for EGVB include rough foods, gastric acid erosion, increased intra-abdominal pressure, and severe coughing. Patients with cirrhosis who have not undergone TIPS should follow a low-salt diet, while those who have undergone TIPS may not need to restrict salt and water intake.
Infection Prevention
Living spaces should be well-ventilated, and good personal hygiene habits should be maintained. Exposure to cold and consumption of unclean food should be avoided.
Etiological Prevention
Patients should understand the causes of cirrhosis and adhere to etiological treatments, such as antiviral medications for hepatitis B. Stable patients should undergo medical follow-ups every 3–6 months, including laboratory tests and imaging studies such as ultrasound, CT, and MRI.
Lifestyle and Work Adjustments
Patients with minimal hepatic encephalopathy, who may have slower reaction times, should avoid driving or working at heights.
Preventing Transmission
Bloodborne transmission of hepatitis B and C should be prevented. Shared use of razors or other potentially invasive personal items should be avoided. Gloves should be worn when handling open wounds of infected individuals. Sexual activity should be moderate, and if no plans for pregnancy exist, the use of condoms is recommended.