Acute pancreatitis (AP) is an inflammatory injury of the pancreas characterized by edema, hemorrhage, and necrosis caused by the pancreas digesting its own tissue due to various etiologies. Clinically, it is marked by acute upper abdominal pain and elevated serum amylase or lipase levels. Most patients experience mild disease with a good prognosis, but a minority may develop multi-organ dysfunction and local pancreatic complications, which are associated with a high mortality rate.
Etiology
Biliary Diseases
Gallstone disease and biliary infections are the primary causes of AP. Since the pancreatic duct and the common bile duct join to form a shared channel that opens into the duodenal ampulla, obstruction of the ampulla by gallstones or ascaris worms, inflammation of the bile ducts, or injury to the sphincter of Oddi caused by migrating stones can impair pancreatic duct outflow and increase intraductal pressure. Small gallstones are particularly likely to cause AP due to their mobility within the biliary system, which complicates clinical diagnosis.
Alcohol
Alcohol stimulates pancreatic juice secretion. When the pancreatic duct cannot adequately drain the large volume of pancreatic juice, intraductal pressure increases, leading to acinar cell damage. The oxidative metabolism of alcohol in the pancreas generates reactive oxygen species, which further activate the inflammatory response. Additionally, alcohol often acts in conjunction with biliary diseases to induce AP.
Pancreatic Duct Obstruction
Pancreatic duct obstruction caused by ductal stones, ascaris worms, strictures, or tumors (e.g., periampullary or pancreatic cancer) can lead to elevated intraductal pressure. Pancreas divisum, a congenital anomaly in which the main and accessory pancreatic ducts fail to fuse during development, results in most pancreatic juice draining through the narrow minor papilla, predisposing to impaired drainage and elevated intraductal pressure.
Diseases of the Descending Duodenum
Inflammatory conditions such as post-bulbar penetrating ulcers or diverticulitis near the duodenal papilla can directly involve the pancreas.
Surgery and Trauma
Abdominal surgeries or blunt abdominal trauma can damage pancreatic tissue, causing severe circulatory disturbances in the pancreas and leading to AP. AP may also occur due to duodenal papilla edema or excessive pressure during contrast injection during ERCP procedures.
Metabolic Disorders
Hypertriglyceridemia may induce or exacerbate AP through microembolism affecting pancreatic microcirculation or the release of toxic free fatty acids from triglyceride breakdown by pancreatic enzymes. Experimental studies suggest that AP is highly likely when serum triglycerides exceed 11.3 mmol/L. Type I hyperlipoproteinemia, often seen in children or non-obese, non-diabetic young adults, can cause recurrent AP due to severe hypertriglyceridemia, known as primary hypertriglyceridemia-induced AP. In obese patients, AP is often accompanied by severe stress and inflammatory responses, leading to rapid increases in triglyceride levels, with peripheral blood samples often showing pronounced lipemia, which exacerbates and accelerates AP progression. Hypercalcemia caused by parathyroid tumors or excessive vitamin D may lead to pancreatic duct calcification and premature activation of pancreatic enzymes, triggering AP.
Medications
Drugs such as thiazide diuretics, azathioprine, glucocorticoids, and sulfonamides may induce AP, often occurring within the first 1–2 months of treatment, without a clear dose-dependent relationship.
Infections and Systemic Inflammatory Responses
AP may follow infections such as mumps, influenza A, Chlamydia pneumoniae, infectious mononucleosis, and Coxsackie virus. It often resolves spontaneously as the infection subsides. During systemic inflammatory responses, the pancreas, as one of the target organs, may also develop acute inflammatory injury.
Overeating
Overeating, especially of fatty foods, is a common trigger for AP, though the threshold for "overeating" varies between individuals and is difficult to quantify. When pancreatic juice cannot flow smoothly into the duodenum due to increased intraductal pressure, AP may develop. While overeating alone is a relatively uncommon cause of AP, it often acts as a precipitating factor, necessitating a thorough search for potential underlying causes.
Other Causes
Rare causes include autoimmune vasculitis, vascular diseases such as major pancreatic vessel embolism, and hereditary or autoimmune-related pancreatitis, which are discussed in the context of chronic pancreatitis. Cases with unknown causes are classified as idiopathic AP.
Pathogenesis
Various causative factors lead to increased intraductal pressure in the pancreas and a significant rise in intracellular Ca2+ levels within acinar cells. This results in premature activation of zymogens (e.g., trypsinogen) by lysosomes within the acinar cells, leading to the autodigestion of pancreatic tissue by large amounts of activated pancreatic enzymes.
Acinar cell damage activates nuclear factor-κB (NF-κB), a key molecule in the inflammatory response. Its downstream inflammatory mediators, such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), arachidonic acid metabolites (prostaglandins, platelet-activating factor), and reactive oxygen species (ROS), increase vascular permeability, resulting in extensive inflammatory exudation.
Pancreatic microcirculatory disturbances contribute to pancreatic hemorrhage and necrosis. Numerous factors involved in the inflammatory process interact in a positive feedback manner, amplifying the inflammation progressively. When the inflammatory response surpasses the body's anti-inflammatory capacity, it spreads systemically, leading to multi-organ inflammatory injury and dysfunction.
Pathology
Acute Inflammatory Lesions of the Pancreas
Acute pancreatitis can be classified into acute edematous pancreatitis and acute hemorrhagic necrotizing pancreatitis. Acute edematous pancreatitis may progress to the hemorrhagic necrotizing form, with the progression occurring over hours to days.
Acute Edematous Pancreatitis
This is more common and involves part or the entirety of the pancreas. The pancreas becomes enlarged, congested, edematous, and infiltrated by inflammatory cells, with minor localized necrosis in some cases.
Acute Hemorrhagic Necrotizing Pancreatitis
This is relatively rare and is characterized by fat necrosis within the pancreas, appearing as gray-white or yellow patches. Severe hemorrhage may cause the pancreas to appear dark brown with fresh hemorrhagic areas. Necrotic foci are surrounded by inflammatory cell infiltration, and phlebitis and thrombosis are often observed.
Local Complications of the Pancreas
Acute Peripancreatic Fluid Collection
During the early stages of AP, exudative fluid accumulates within and around the pancreas without fibrous encapsulation. This fluid may appear as single or multiple pockets, and approximately half of the cases resolve spontaneously during the disease course.
Pancreatic Fistula
Inflammation in the pancreas can cause pancreatic duct rupture, leading to the leakage of pancreatic juice, which constitutes a pancreatic fistula. Internal pancreatic fistulas are often the cause of refractory pancreatic pseudocysts and pancreatic pleural or peritoneal effusions. External pancreatic fistulas occur when pancreatic juice drains externally through abdominal drainage tubes or surgical incisions.
Pancreatic Pseudocysts and Pancreatic Pleural/Peritoneal Effusions
Exudative fluid containing pancreatic fistulas may accumulate and become difficult to resorb. After approximately one month, fibrous tissue proliferation leads to the formation of a cyst wall, resulting in pancreatic pseudocysts. These pseudocysts vary in shape and size and differ from true cysts in that their walls lack epithelial lining, being composed of granulation or fibrous tissue. The cyst contents are sterile and contain pancreatic enzymes. Extensive inflammatory exudation combined with pancreatic fistulas can also lead to pancreatic pleural or peritoneal effusions.
Pancreatic Necrosis
The likelihood of isolated pancreatic parenchymal necrosis, peripancreatic fat necrosis, and combined pancreatic parenchymal and peripancreatic fat necrosis is approximately 5%, 20%, and 75%, respectively. In the early stages, acute necrotic collections (ANC) contain both solid and liquid components and typically lack clear boundaries. After about one month, as omental wrapping and fibrous tissue proliferation occur, these necrotic materials become encapsulated, forming walled-off necrosis (WON).
Pancreatic Abscess
Infection of peripancreatic fluid collections, pancreatic pseudocysts, or pancreatic necrosis may lead to the formation of abscesses.
Left-Sided Portal Hypertension (LSPH)
Severe pancreatic necrosis, extensive exudation, persistent pseudocyst compression, and chronic inflammation may result in splenic vein thrombosis, leading to splenomegaly and gastric fundal varices.
Pathology of Multi-Organ Inflammatory Injury Caused by AP
Systemic inflammatory responses can affect other organs, such as the small intestine, lungs, liver, and kidneys, resulting in acute inflammatory pathological changes in these organs.
Clinical Manifestations
The clinical presentation of acute pancreatitis (AP) varies depending on the severity of the disease.
Acute Abdominal Pain
Acute abdominal pain is the most common initial symptom, often severe and typically located in the mid to left upper abdomen or even throughout the abdomen. In some cases, the pain radiates to the back. At the onset of the disease, patients may experience nausea, emesis, and mild fever. Common physical signs include tenderness in the epigastrium, reduced bowel sounds, and mild dehydration.
Acute Multi-Organ Dysfunction and Failure
On the basis of the aforementioned symptoms, persistent and unrelieved abdominal pain, progressively worsening abdominal distension, and subsequent dysfunction or failure of the circulatory, respiratory, intestinal, renal, and hepatic systems may occur. Table 1 outlines some symptoms and signs of multi-organ dysfunction.
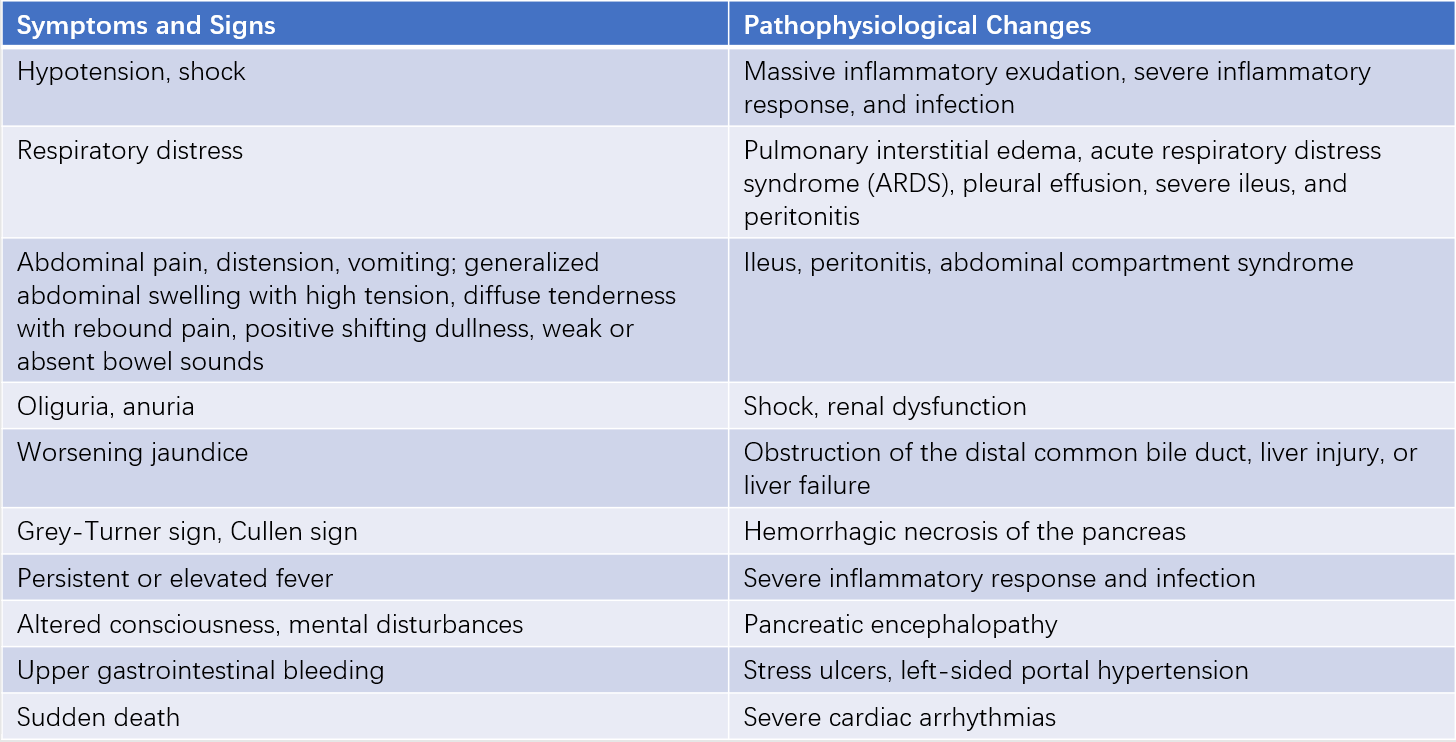
Table 1 Symptoms, signs, and corresponding pathophysiological changes in AP-associated multiple organ dysfunction
Local Complications of the Pancreas
In cases of acute fluid collection, pancreatic necrosis, or pancreatic ascites, patients often exhibit significant abdominal pain and distension. Rapid disease progression may lead to shock and abdominal compartment syndrome. Large volumes of pancreatic pleural effusion can cause respiratory distress. The presence of pleural effusion in the early stages of the disease suggests a higher likelihood of developing severe acute pancreatitis.
When pancreatic necrosis results in significant and sustained hemorrhage, patients may present with refractory shock. Hemorrhagic ascites, aided by pancreatic enzymes, can infiltrate the subcutaneous tissue, leading to Grey-Turner’s sign (bruising on the flanks) or Cullen’s sign (bruising around the umbilicus).
Pancreatic pseudocysts smaller than 5 cm have a spontaneous resorption rate of approximately 50% within six weeks. Larger pseudocysts may cause significant abdominal distension and symptoms of upper or mid-gastrointestinal obstruction. The progression from acute necrotic collection (ANC) to walled-off necrosis (WON) may involve sterile or infected lesions. When pancreatic parenchymal necrosis exceeds 30%, the likelihood of infection increases significantly. Pancreatic infections typically occur two weeks after the onset of AP, though in rare cases, infection may develop within the first week. Signs of infection include:
- Body temperature >38.5°C and a white blood cell count >16×109/L.
- Peritoneal irritation signs involving more than two abdominal quadrants; infections in the retroperitoneal space may manifest as significant lumbar tenderness, lumbar fullness, redness of the skin, or pitting edema.
- CT imaging showing gas bubbles within ANC or WON.
Patients with pancreatic abscesses often present with prolonged disease courses, fever, abdominal pain, weight loss, and malnutrition.
After recovery from pancreatic necrosis, exocrine pancreatic insufficiency may develop depending on the extent of necrosis. Symptoms include food intolerance, postprandial abdominal distension and pain, reduced appetite, persistent mild diarrhea, steatorrhea, and malnutrition.
Left-sided portal hypertension (LSPH) can occur in the early stages of severe AP and is usually transient, resolving as pancreatic and peripancreatic inflammation subsides. When inflammation persists and is accompanied by complications such as pseudocysts or abscesses, LSPH becomes difficult to reverse. Patients may develop gastric fundal varices, leading to melena, hematemesis, or even fatal gastrointestinal bleeding.
Auxiliary Examinations
Key Serum Markers for Diagnosing AP
Amylase
Serum amylase levels start to rise 2–12 hours after the onset of AP, peak at 48 hours, and return to normal within 3–5 days. Since salivary glands also produce amylase, elevated serum amylase levels in the absence of acute abdominal symptoms may originate from the salivary glands. Amylase is excreted by the kidneys, and urinary amylase levels increase during AP. However, mild renal dysfunction can affect the accuracy and specificity of urinary amylase measurements, limiting its clinical diagnostic value.
Lipase
Serum lipase levels begin to rise 24–72 hours after the onset of AP and remain elevated for 7–10 days. Lipase is slightly more sensitive and specific than amylase. The levels of serum amylase and lipase do not correlate directly with disease severity, and in some cases of severe AP, pancreatic enzyme levels may remain normal or only mildly elevated. In pancreatic pleural effusion, ascites, or pseudocyst fluid, the levels of these two enzymes are often significantly elevated.
Laboratory Indicators Reflecting Pathophysiological Changes in AP
Laboratory tests can provide information about systemic inflammation, organ dysfunction, and metabolic disturbances associated with AP.
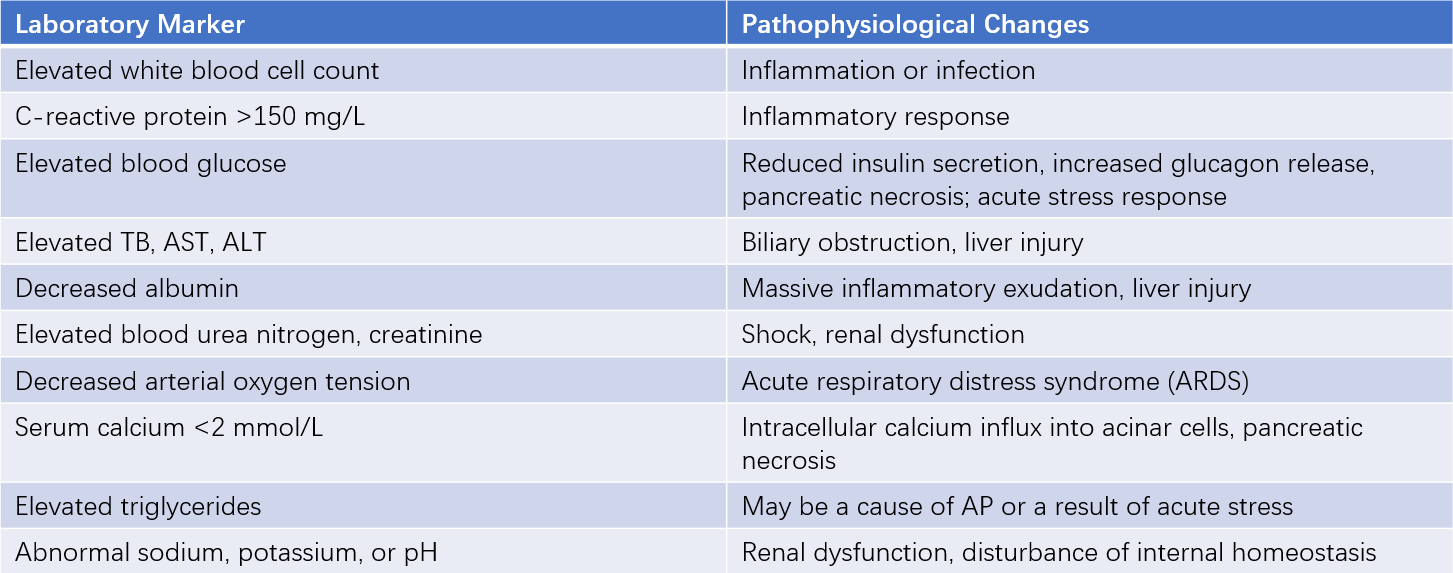
Table 2 Laboratory markers reflecting pathophysiological changes in AP
Imaging Changes in the Pancreas and Other Organs
Abdominal Ultrasound
Abdominal ultrasound is a routine initial screening tool for AP. However, it is often limited by interference from gastrointestinal gas, making detailed observation of pancreatic morphology challenging. Ultrasound is useful for assessing the gallbladder and bile ducts, serving as a preliminary screening method for biliary causes of pancreatitis. It is also commonly used to diagnose, monitor, and guide puncture of pancreatic pseudocysts.
Abdominal CT and MRI
Non-contrast CT was initially used to diagnose pancreatitis and is helpful for assessing peripancreatic inflammatory changes and pleural or abdominal effusions. Contrast-enhanced CT can evaluate the extent of pancreatic necrosis and is typically performed about one week after disease onset. MRI is particularly useful for identifying biliary abnormalities and peripancreatic fluid collections.
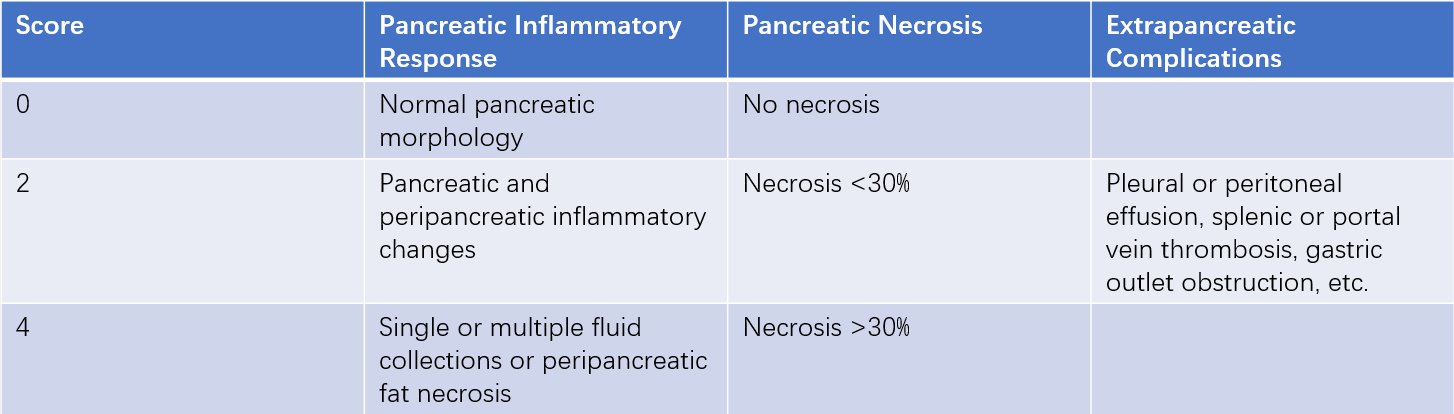
Table 3 Acute pancreatitis CT scoring system
Note: A score of ≥4 indicates MSAP or SAP.
Endoscopic Ultrasound (EUS)
EUS is valuable for assessing biliary conditions and can help avoid unnecessary ERCP interventions. It is also helpful for evaluating local pancreatic lesions and determining the underlying cause of pancreatitis.
Diagnosis
As a common cause of acute abdomen, the diagnostic process for acute pancreatitis (AP) includes the following:
Confirmation of AP Diagnosis
A diagnosis of AP requires the presence of at least two of the following three criteria:
- Acute, persistent epigastric pain.
- Serum amylase or lipase levels exceeding three times the upper limit of normal.
- Typical imaging findings of AP.
This diagnosis should generally be established within 48 hours after patient admission.
Assessment of AP Severity
The severity of AP is classified into four categories based on the presence of organ failure (OF), pancreatic necrosis, and pancreatic infection:
- Mild acute pancreatitis (MAP).
- Moderately severe acute pancreatitis (MSAP).
- Severe acute pancreatitis (SAP).
- Critical acute pancreatitis (CAP).
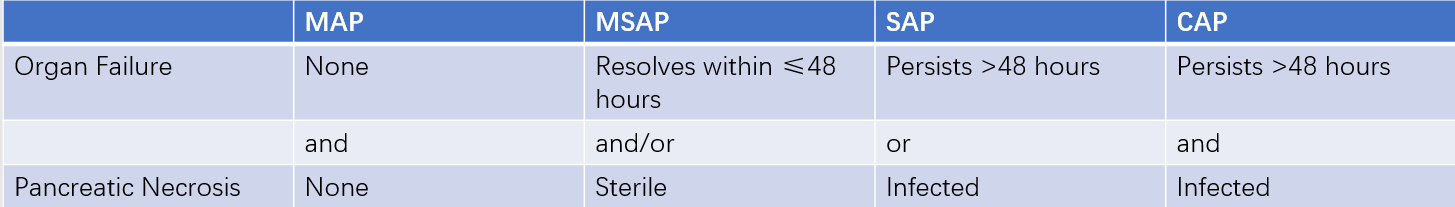
Table 4 Classification of AP severity
Organ failure is evaluated using quantitative indicators for respiratory, circulatory, and renal function. An organ score of ≥2 indicates organ failure. Intestinal failure is characterized by abdominal compartment syndrome. Acute liver failure is defined as the occurrence of hepatic encephalopathy in two or more phases of the disease course, accompanied by:
- Severe fatigue, significant loss of appetite, abdominal distension, nausea, and vomiting.
- Progressive worsening of jaundice over a short period.
- Pronounced bleeding tendency with prothrombin activity ≤40% (or INR ≥1.5) after excluding other causes.
- Progressive reduction in liver size.
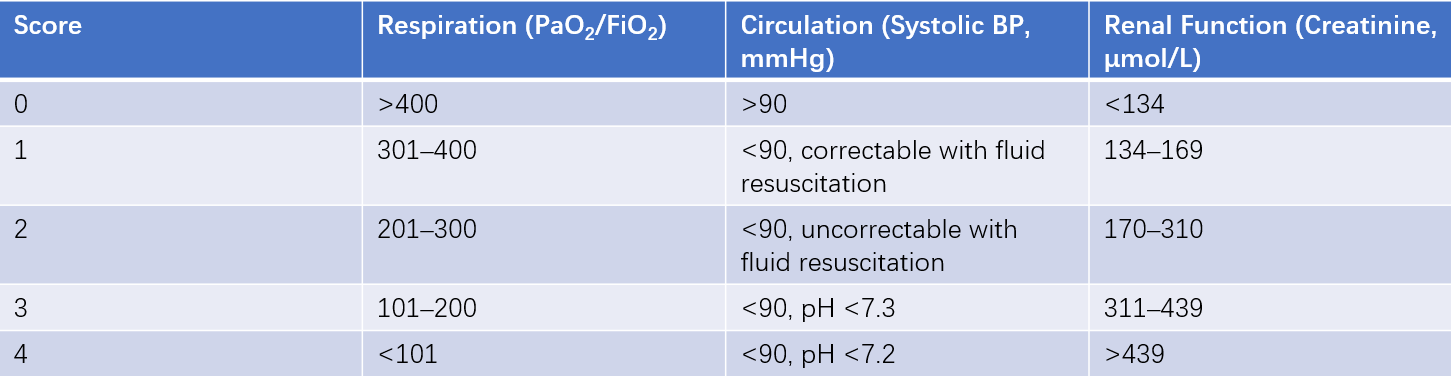
Table 5 Modified Marshall scoring system for organ dysfunction
Note:
PaO2: Arterial oxygen tension (normal range: 95–100 mmHg).
FiO2: Fraction of inspired oxygen (air: 21%, pure oxygen at 2 L/min: 25%, 4 L/min: 30%, 6–8 L/min: 40%, 9–10 L/min: 50%).
Pancreatic infection is typically diagnosed based on clinical manifestations and laboratory findings. When clinical evidence is insufficient but suspicion remains high, fine-needle aspiration of pancreatic or peripancreatic tissue under CT or ultrasound guidance can be performed for bacterial smear or culture.
Identification of the Etiology
Efforts should aim to identify the cause of AP in over 80% of patients during hospitalization. Early resolution of the underlying cause helps shorten the disease course, prevent SAP, and avoid future recurrence. Biliary diseases remain the leading cause of AP, and attention should be paid to the possibility of multiple etiological factors. While CT is primarily used to assess the severity of AP, MRCP is recommended for investigating biliary and pancreatic duct abnormalities.
Differential Diagnosis
Acute pancreatitis must often be differentiated from conditions such as cholelithiasis, peptic ulcer disease, myocardial infarction, and acute intestinal obstruction, as detailed in relevant sections. In these conditions, serum amylase and lipase levels may also be elevated but are typically less than twice the normal upper limit.
Treatment
The management of AP focuses on two primary objectives:
- Identifying and eliminating the underlying cause.
- Controlling the inflammatory response.
Even in cases of SAP, conservative and minimally invasive treatments are preferred whenever possible. Clinical evidence suggests that surgical trauma in SAP exacerbates the systemic inflammatory response and increases mortality. For biliary AP, endoscopic treatment during the current hospitalization or elective cholecystectomy after recovery is recommended to prevent recurrence. Local pancreatic complications, such as symptomatic pancreatic pseudocysts, pancreatic abscesses, or left-sided portal hypertension, may require endoscopic or surgical intervention.
Monitoring
The progression from inflammatory response to organ dysfunction and eventual organ failure can vary in duration and severity, necessitating close monitoring. Disease progression should be assessed based on symptoms, physical signs, laboratory tests, and imaging findings. Elderly patients, those with obesity (BMI ≥28 kg/m2), and pregnant women are considered high-risk groups for SAP. The APACHE II scoring system is useful for dynamic evaluation of disease severity. This system includes acute physiology scores, age scores, and chronic health scores, quantifying multiple physiological parameters to assess the severity of acute illness.
The scoring process involves downloading APACHE II scoring software and inputting parameters that are readily available in most hospitals. An APACHE II score ≥8 indicates a 70% likelihood of developing SAP and identifies the patient as being at high risk for SAP.
Organ Support
Fluid Resuscitation
Fluid resuscitation aims to rapidly correct tissue hypoxia and is an essential measure for maintaining blood volume as well as water and electrolyte balance. If circulatory dysfunction is present, the first 24 hours after onset is considered the golden period for fluid resuscitation. For patients with moderately severe acute pancreatitis (MSAP) without significant blood loss, the daily fluid volume should generally be controlled at 3,500–4,000 ml. When performing fluid resuscitation with crystalloids, balanced solutions such as lactated Ringer's solution should be supplemented, while avoiding excessive use of normal saline to prevent chloride overload. Metabolic acidosis, commonly caused by lactate accumulation due to hypoxia, should be addressed by supplementing sodium bicarbonate. In severe cases, where significant pancreatic exudation leads to protein loss, albumin supplementation is necessary to maintain organ function effectively. Although fluid volume and rate can be adjusted based on central venous pressure, this parameter may be less reliable in acute pancreatitis (AP) due to significant abdominal distension and paralytic ileus. In severe acute pancreatitis (SAP), the fluid volume should be adjusted daily, and excessive fluid administration should be avoided. Clinical indicators for monitoring fluid resuscitation include heart rate, respiratory rate, blood pressure, urine output, blood gas analysis, pH levels, blood urea nitrogen, and creatinine.
Respiratory Function
For mild cases, oxygen can be administered via nasal cannula or face mask with the goal of maintaining arterial oxygen saturation above 95%. In cases of acute lung injury or respiratory distress, positive pressure mechanical ventilation may be required. Fluid volume should be limited to less than 2,000 ml in such situations, and diuretics may be used as needed, guided by parameters such as urine output, blood pressure, and arterial blood pH.
Maintaining Intestinal Function
Laxatives and oral antibiotics can help reduce bacterial translocation and intestinal inflammation in cases of impaired intestinal barrier function. Laxatives reduce bacterial overgrowth in the intestinal lumen, promote peristalsis, and help maintain the intestinal mucosal barrier. Sodium sulfate (40 g dissolved in 600 ml of warm water) can be administered in divided doses. After bowel evacuation, lactulose can be used to maintain bowel movements once every 1–2 days. Oral antibiotics such as levofloxacin (0.5 g once daily) combined with metronidazole (0.2 g three times daily) can be administered for a course of four days. Gastrointestinal decompression may alleviate abdominal distension and can be employed when necessary.
Continuous Blood Purification
In cases of refractory acute renal failure, continuous blood purification can remove harmful metabolic products or exogenous toxins through selective or non-selective adsorption, thereby achieving blood purification. Early use in SAP may help clear inflammatory mediators, improving and restoring the function of vital organs such as the lungs, kidneys, and brain, and preventing further disease progression.
Reducing Pancreatic Secretion
Fasting
Food is a natural stimulant for pancreatic secretion. Short-term fasting after the onset of AP can reduce pancreatic secretion and minimize the autodigestive effects of pancreatic enzymes on the pancreas. Resting the pancreas has historically been the theoretical basis for treating AP. However, during AP, acinar cells undergo widespread apoptosis or necrosis, severely impairing exocrine function, and the therapeutic benefit of fasting to suppress pancreatic secretion is limited. Fasting during the first 48 hours may help alleviate abdominal distension and pain.
Somatostatin and Its Analogues
Somatostatin, synthesized by D cells in the gastrointestinal mucosa, inhibits the basal secretion of pancreatic juice stimulated by secretin and cholecystokinin.
Controlling Inflammation
Fluid Resuscitation
Successful fluid resuscitation is one of the key early measures to control the systemic inflammatory response caused by AP.
Somatostatin
Somatostatin is an important anti-inflammatory peptide in the body. During AP, circulating and intestinal mucosal levels of somatostatin decrease significantly, exacerbating pancreatic and systemic inflammatory responses. Supplementation with exogenous somatostatin or its analogue octreotide not only inhibits pancreatic secretion but also helps control pancreatic and systemic inflammation. For mild cases, somatostatin (250 μg/h) or octreotide (25 μg/h) can be administered via continuous intravenous infusion for three days during the early stages of the disease. For patients at high risk of SAP or those with MSAP, somatostatin (500 μg/h) or octreotide (50 μg/h) should be administered within 48 hours after onset. After 3–4 days, the dosage can be reduced to 250 μg/h or 25 μg/h, with a total treatment duration of 4–5 days. This approach not only helps prevent the development of SAP but also alleviates SAP to some extent.
Early Enteral Nutrition
The gastrointestinal tract is a key source of the systemic inflammatory response. Early enteral nutrition helps control systemic inflammation.
Pain Management
For most patients, abdominal pain can be significantly relieved after intravenous infusion of somatostatin or octreotide. For severe pain, intramuscular injection of pethidine (50–100 mg per dose) may be used. Morphine is not recommended due to its tendency to increase sphincter of Oddi pressure, and anticholinergic agents such as atropine should be avoided as they can induce or exacerbate paralytic ileus.
Emergency Endoscopic Treatment to Address the Underlying Cause
For biliary AP caused by common bile duct obstruction, acute suppurative cholangitis, or biliary sepsis, early endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy, stone extraction, or nasobiliary drainage is recommended. This approach helps reduce intrapancreatic ductal pressure, rapidly controls pancreatic inflammation and infection, and achieves effective, minimally invasive, etiological treatment. This method is associated with confirmed efficacy, minimal trauma, rapid symptom relief, improved prognosis, shortened disease course, reduced treatment costs, and prevention of AP recurrence.
Prevention and Anti-Infection Measures
Acute pancreatitis (AP) is primarily a chemical inflammation, but it is highly prone to infection during its course, which is a key factor in the progression to severe disease and even death. The primary source of infection is the intestine. Measures to prevent pancreatic infection include:
- The use of laxatives and oral antibiotics, as previously described.
- Early restoration of enteral nutrition, which facilitates the repair of damaged intestinal mucosa and reduces bacterial translocation.
- When pancreatic necrosis exceeds 30%, the risk of pancreatic infection increases. Prophylactic intravenous administration of imipenem or meropenem for 7–10 days may help reduce secondary infections of necrotic pancreatic tissue.
When pancreatic infection is suspected or confirmed, antibiotics that target Gram-negative bacteria and anaerobes and can penetrate the blood-pancreas barrier should be selected. These include carbapenems, third-generation cephalosporins combined with anti-anaerobic agents, or quinolones combined with anti-anaerobic agents. The treatment course typically lasts 7–14 days, and a step-down strategy is recommended for antibiotic selection. As AP progresses, the bacterial spectrum of pancreatic infections may change, shifting from predominantly single organisms and Gram-negative bacteria (mainly Escherichia coli) to multiple organisms and Gram-positive bacteria. Additionally, empirical antifungal therapy may be considered if fungal infection is suspected.
Early Enteral Nutrition
The goal of early enteral nutrition is to improve the gastrointestinal mucosal barrier, reduce inflammatory responses, and prevent bacterial translocation and pancreatic infection. For patients with mild acute pancreatitis (MAP) or moderately severe acute pancreatitis (MSAP) who receive timely and effective treatment, enteral nutrition can generally be initiated orally within 48–72 hours after disease onset. If significant abdominal distension prevents enteral nutrition, it can be resumed once emesis subsides and the intestinal tract becomes unobstructed. Dietary restoration should begin with small amounts of easily digestible carbohydrate-based foods, supplemented with digestive enzymes, and gradually increase to include small amounts of protein until normal dietary intake is restored. For patients with prolonged disease courses complicated by large pancreatic pseudocysts or walled-off necrosis (WON) causing partial upper gastrointestinal obstruction, endoscopic gastrostomy and placement of a jejunal feeding tube can be performed to ensure enteral nutrition.
Elective Endoscopic, Laparoscopic, or Surgical Management of Underlying Causes
Underlying conditions such as common bile duct stones, pancreas divisum, congenital pancreatic duct stenosis, gallstones, chronic pancreatitis, periampullary cancer, and pancreatic cancer are often addressed through elective surgery after recovery from AP. Whenever possible, minimally invasive approaches are preferred.
Local Pancreatic Complications
Pancreatic Pseudocysts
Pseudocysts smaller than 4 cm are often spontaneously resorbed. Pseudocysts larger than 6 cm or multiple pseudocysts are less likely to resolve spontaneously. If no reduction or resorption is observed after 6–8 weeks of observation, drainage may be required. Drainage methods include percutaneous drainage, endoscopic drainage, or surgical drainage.
Management of Pancreatic Abscesses
If a pancreatic abscess does not resolve following adequate antibiotic therapy, abdominal drainage or lavage may be performed. If infection remains uncontrolled, necrosectomy and drainage surgery may be necessary.
Patient Education
Patients should be informed of the presence of risk factors for severe acute pancreatitis (SAP) and the potential for adverse outcomes during the early stages of AP.
Determining the cause of AP should be emphasized, and patient cooperation in history-taking, diagnosis, and treatment should be encouraged.
The therapeutic role of endoscopic retrograde cholangiopancreatography (ERCP) in the management of AP should be explained.
The necessity of mechanical ventilation or continuous blood purification should be communicated.
The importance and key points of implementing enteral nutrition should be explained.
For patients with local complications, guidance on regular follow-up after discharge should be provided.
Prognosis
Mild cases of AP often recover within approximately one week without leaving sequelae. The mortality rate for severe cases is approximately 15%. Patients who survive organ failure following intensive treatment often experience complications such as pancreatic pseudocysts, walled-off necrosis (WON), pancreatic abscesses, and splenic vein thrombosis, along with varying degrees of pancreatic insufficiency. Patients who do not address the underlying cause may experience recurrent AP, which, with repeated inflammation and fibrosis, may progress to chronic pancreatitis.
Prevention
Effective management of biliary and pancreatic diseases, moderation in alcohol consumption and food intake, and strict abstinence from alcohol for some patients are important preventive measures.