Pulmonary tuberculosis (TB) remains a major infectious disease that seriously threatens human health in the 21st century. It is a global public health and social issue and one of the key diseases targeted for control.
Since the 1980s, the tuberculosis epidemic has shown a significant resurgence and a global worsening trend. However, in the 21st century, the global TB epidemic has slowly declined, with the incidence of TB decreasing from 2005 to 2019. Nevertheless, in 2021, the global incidence of TB increased by 3.6% compared to 2020, reversing the downward trend.
Epidemiology
Globally, 1/3 of the population (approximately 2 billion people) has been infected with Mycobacterium tuberculosis. The prevalence of TB is closely associated with economic conditions, with high TB prevalence corresponding to low GDP levels.
In 2021, there were approximately 6.4 million new TB cases worldwide, including 400,000 new cases among HIV-infected individuals (6.7%). About 1.6 million people died from TB, of whom 187,000 were HIV-infected. Additionally, approximately 450,000 new cases of rifampin-resistant TB were reported globally in 2021. The treatment success rate for drug-resistant TB remains relatively low, at around 60%.
Mycobacterium Tuberculosis
The causative agent of TB is the Mycobacterium tuberculosis complex, which includes Mycobacterium tuberculosis (MTB), Mycobacterium bovis, Mycobacterium africanum, and Mycobacterium microti. Over 90% of human pulmonary TB cases are caused by Mycobacterium tuberculosis.
Typical Mycobacterium tuberculosis is a slender, slightly curved rod with rounded ends. In sputum samples, it may appear in various shapes, including T, V, Y, filamentous, spherical, or rod-like forms. Mycobacterium tuberculosis stains red with acid-fast staining and resists decolorization by acid-alcohol, hence the term acid-fast bacilli.
The bacterium is highly resistant to drying, cold, acids, and alkalis but is sensitive to ultraviolet (UV) light. Under direct sunlight, Mycobacterium tuberculosis in sputum can be killed within 2-7 hours. In laboratories or hospital wards, UV lamps are commonly used for disinfection. A 10W UV lamp placed 0.5-1 meter from the target for 30 minutes has significant bactericidal effects.
Mycobacterium tuberculosis is an obligate aerobe with high nutritional requirements. Its generation time is 14-20 hours, and it typically requires 2-8 weeks for culture growth. The bacterium's cell wall is complex, primarily composed of lipids, proteins, and polysaccharides. Lipids account for 50%-60% of the total, with waxes comprising about 50%. These lipids are associated with tissue necrosis, caseous liquefaction, cavity formation, and hypersensitivity reactions in TB. Bacterial proteins, which exist in bound forms, are the main components of tuberculin and induce delayed-type hypersensitivity. Polysaccharides are involved in immune responses, such as serum reactions.
Transmission of Tuberculosis among Humans
The main source of TB infection is patients with active TB, particularly those with smear-positive sputum. Mycobacterium tuberculosis is primarily transmitted through the respiratory tract, with droplet transmission being the most important route. Healthy individuals can become infected by inhaling droplets containing the bacteria expelled when an infected person coughs or sneezes. Transmission through the digestive tract or skin is now rare.
The degree of infectivity depends on the amount of Mycobacterium tuberculosis expelled by the patient, the density of aerosolized bacteria in the air, ventilation conditions, the closeness and duration of contact, and the immune status of the individual. Ventilation and reducing the density of airborne bacteria are effective measures to reduce TB transmission.
Whether infection leads to disease depends on the host's immune status. A weakened immune system is a major risk factor for TB development. Key factors that compromise immunity include diabetes, HIV infection, alcohol abuse, smoking, and malnutrition. Other risk factors, such as COVID-19, silicosis, anti-TNF-α therapy, dialysis, and the use of immunosuppressants, also increase the risk of TB in high-risk populations.
Development of Tuberculosis in the Human Body
Primary Infection
After the first inhalation of aerosols containing Mycobacterium tuberculosis, infection depends on the virulence of the bacteria and the innate phagocytic and bactericidal ability of alveolar macrophages. Components such as lipids in Mycobacterium tuberculosis can resist destruction by lysosomal enzymes. If the bacteria survive and proliferate inside and outside alveolar macrophages, the affected lung tissue develops inflammation, forming a primary lesion.
Mycobacterium tuberculosis from the primary lesion can spread through lymphatic drainage to the hilar lymph nodes, causing lymph node enlargement. The combination of the primary lesion and the enlarged tracheobronchial lymph nodes is known as the primary complex. The primary lesion may expand further, spreading directly or via the bloodstream to nearby tissues and organs, leading to TB.
Once Mycobacterium tuberculosis begins to proliferate in the body, the immune system mounts a specific response through cell-mediated immunity. This response halts bacterial proliferation in the primary lesion, hilar lymph nodes, and disseminated sites, leading to rapid resolution of inflammation or residual calcified lesions. Enlarged lymph nodes gradually shrink, fibrose, or calcify, and most disseminated bacteria are eliminated. This is the most common benign outcome of primary infection. However, a small number of bacteria may remain dormant, serving as a potential source of secondary TB.
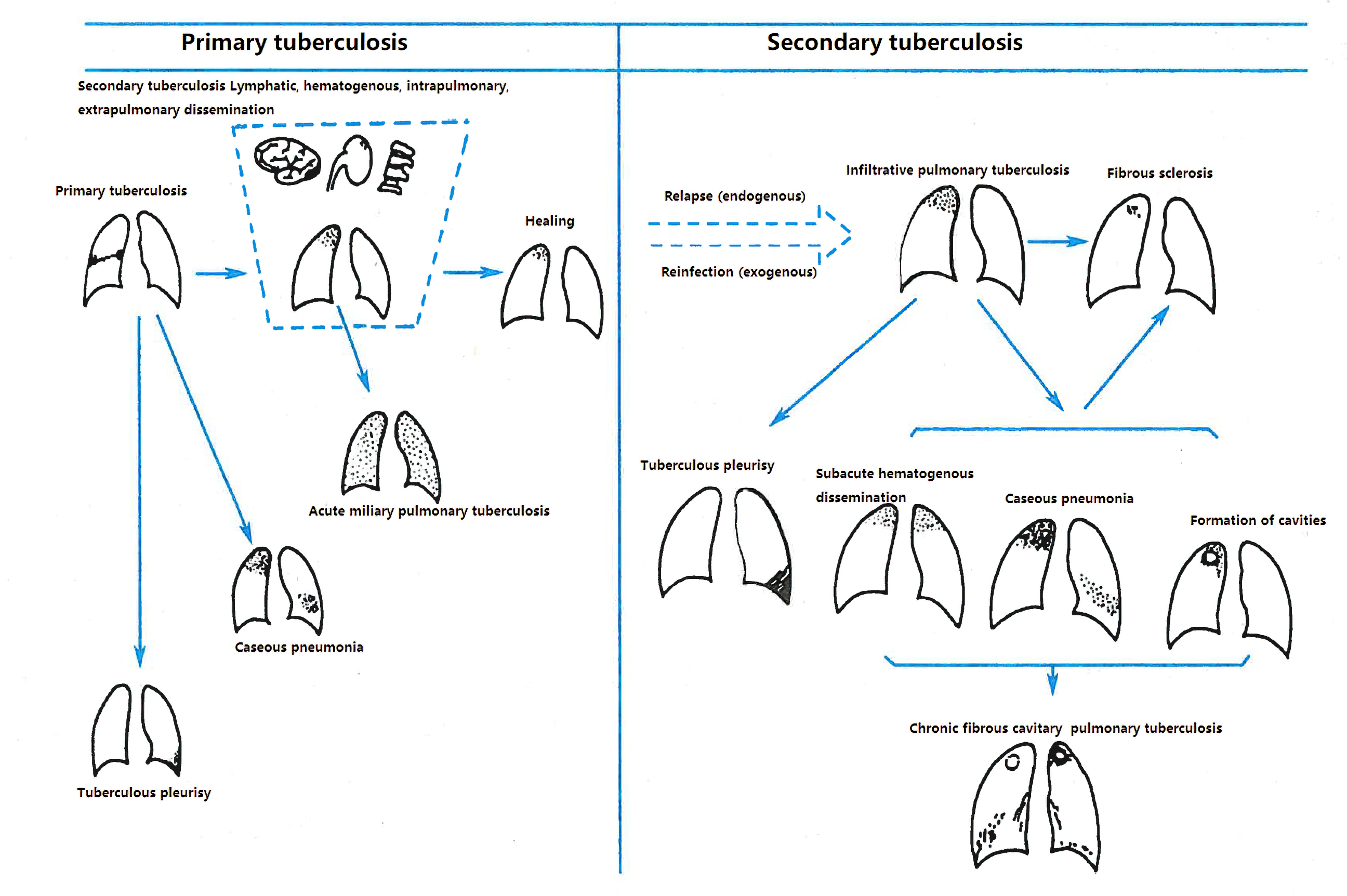
Figure 1 Schematic diagram of the natural course of pulmonary tuberculosis
Immunity and Delayed-type Hypersensitivity in Tuberculosis
Mycobacterium tuberculosis is an intracellular pathogen, and the primary protective immune mechanism against TB is T-cell-mediated cellular immunity. Humoral immunity plays a minimal role in controlling the infection.
In innate immunity, macrophages are the main target cells for TB infection and the most representative cells in the host's defense against the disease. Alveolar macrophages secrete large amounts of cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α, recruiting monocytes and other immune cells to the site of infection. This leads to the formation of granulomas, which limit bacterial spread and kill the bacteria.
Mycobacterium tuberculosis antigens are presented to CD4+ T cells via MHC class II molecules on macrophages. CD4+ T cells promote immune responses and differentiate into Th1 cells under early cytokines such as IL-12 and IL-18. These Th1 cells produce large amounts of interferon-γ, further activating macrophages to enhance phagocytosis and bacterial killing. CD4+ T cells also contribute to the apoptosis of infected cells. Meanwhile, CD8+ T cells play a role by producing granulysin and perforin, which directly kill Mycobacterium tuberculosis.
The immune protection mechanisms in TB are highly complex, and many aspects remain under investigation.
Secondary Tuberculosis
Secondary TB differs significantly from primary TB. It often presents with pronounced clinical symptoms, cavity formation, and bacterial shedding, making it highly infectious.
Secondary TB can arise in two ways:
- Endogenous reactivation: Dormant bacteria from a latent primary infection become reactivated, leading to disease. It is estimated that about 10% of individuals infected with Mycobacterium tuberculosis develop secondary TB at some point in their lifetime.
- Exogenous reinfection: New exposure to Mycobacterium tuberculosis leads to reinfection and disease.
The predominant mode of secondary TB development depends on the local epidemiology and severity of the TB epidemic.
Pathology
Basic Pathological Changes
The fundamental pathological changes in tuberculosis (TB) include inflammatory exudation, proliferation, and caseous necrosis. The pathological process of TB is characterized by simultaneous destruction and repair, so these three pathological changes often coexist. However, one type of change may dominate, and transitions between the three types can occur.
Exudative Lesions
These are predominant in the early stages of TB inflammation or during disease relapse and progression. They are characterized by local infiltration of neutrophils, which are subsequently replaced by macrophages and lymphocytes.
Proliferative Lesions
These occur during the recovery phase when the host's resistance is stronger. They are marked by the formation of typical tubercles, approximately 0.1 mm in diameter, which may merge into visible nodules. Tuberculosis nodules are composed of lymphocytes, epithelioid cells, Langhans giant cells, and fibroblasts, with caseous necrosis sometimes present in the center. Langhans giant cells are multinucleated cells formed by the aggregation and fusion of numerous epithelioid cells.
Caseous Necrosis
This type of lesion is predominant in cases where Mycobacterium tuberculosis has strong virulence, a high bacterial load, heightened hypersensitivity reactions, or weakened host resistance. Microscopically, caseous necrosis appears as red-staining, structureless granular material rich in lipids. Macroscopically, it appears pale yellow and cheese-like, hence the term caseous necrosis.
Pathological Outcomes
Before the advent of anti-TB chemotherapy, the pathological outcomes of TB were characterized by slow resorption and healing, frequent relapses, and dissemination. With chemotherapy, early exudative lesions can completely resolve or leave only minimal fibrous strands. Some proliferative lesions or smaller caseous necrotic lesions may shrink, gradually fibrose, or become encapsulated by proliferating fibrous tissue, forming scattered small nodules.
Without chemotherapy, caseous necrotic lesions often undergo liquefaction necrosis or form cavities. The liquefied material, rich in Mycobacterium tuberculosis, can spread through the bronchi to other parts of the ipsilateral lung or the contralateral lung, leading to new lesions. With chemotherapy, most Mycobacterium tuberculosis in caseous lesions are killed, and the lesions gradually shrink, resolve, or calcify.
Clinical Manifestations
The clinical manifestations of pulmonary tuberculosis vary but share common features.
Symptoms
Persistent cough and expectoration for more than two weeks, or hemoptysis, are common suspicious symptoms of pulmonary TB. The cough is typically mild, with dry cough or scanty mucus sputum. When cavities form, sputum production increases, and if secondary bacterial infections occur, the sputum may become purulent. In cases of bronchial TB, symptoms include an irritating cough. About 1/3 of patients experience hemoptysis, which is usually mild but can occasionally be massive.
When TB lesions involve the pleura, pleuritic chest pain may occur, which worsens with respiratory movements and coughing.
Dyspnea is common in patients with caseous pneumonia or large pleural effusions.
Fever is the most common systemic symptom, often presenting as prolonged low-grade fever in the afternoon or evening, with a return to normal temperature by morning.
Patients may experience fatigue, diaphoresis, anorexia, and emaciation. Women of childbearing age may have menstrual irregularities.
Signs
The physical signs vary depending on the nature and extent of the lesions.
In cases of limited lesions, there may be no detectable physical signs.
Extensive exudative lesions or caseous necrosis may present with signs of lung consolidation.
Tuberculous pleuritis may manifest as signs of pleural effusion, including tracheal deviation toward the unaffected side, fullness of the affected hemithorax on inspection, decreased tactile fremitus, dullness on percussion, and absent breath sounds on auscultation.
Bronchial TB may present with localized wheezing sounds.
A small number of patients may exhibit rheumatic fever-like symptoms, referred to as tuberculous rheumatism. This is more common in adolescent females and often involves the large joints of the limbs. Erythema nodosum or annular erythema may intermittently appear near the affected joints.
Diagnosis
Diagnostic Principles
The diagnosis of pulmonary tuberculosis is primarily based on microbiological evidence (including bacteriological and molecular biological tests). It requires a comprehensive analysis of epidemiological history, clinical manifestations, chest imaging, and related auxiliary tests.
For pediatric TB, in addition to sputum microbiological testing, the examination of gastric aspirates for microbiological evidence is also crucial.
Diagnostic Methods
Imaging Studies
Chest imaging is an essential tool for the early detection of tuberculosis (TB). The characteristic imaging features of pulmonary TB often involve the apical-posterior segment of the upper lobe, the dorsal segment of the lower lobe, and the posterior basal segment. The lesions are polymorphic, with infiltration, proliferation, caseation, and fibrotic calcification often coexisting.
Chest X-ray is the first-line routine method for diagnosing pulmonary TB. It can detect early and subtle TB lesions and determine the extent, location, morphology, density, and relationship of the lesions with surrounding tissues. It can also identify associated imaging signs, assess the nature of the lesion, detect activity, and evaluate the presence, size, and characteristics of cavities.
Chest CT provides higher resolution and allows for the evaluation of fine lesion features. It can detect early miliary opacities and reduce missed diagnoses of small lesions. CT can clearly display the characteristics and nature of different types of pulmonary TB lesions, their relationship with the bronchi, the presence of cavities, and changes indicating progression or improvement. It can also accurately reveal mediastinal lymph node enlargement. CT is commonly used for diagnosing pulmonary TB, differentiating it from other chest diseases, and guiding procedures such as biopsy, drainage, and interventional treatments.
Detection of Mycobacterium Tuberculosis in Sputum
Sputum examination for Mycobacterium tuberculosis is the primary method for confirming pulmonary TB. It is also the main basis for formulating therapy regimens and assessing treatment efficacy. All patients with suspected TB symptoms or abnormal pulmonary opacities must undergo sputum testing.
Since Mycobacterium tuberculosis shedding is intermittent and uneven, multiple sputum samples are required. For newly diagnosed patients, at least three sputum samples should be submitted (morning, nighttime, and on-the-spot sputum). For follow-up patients, two sputum samples are sufficient each time. For patients unable to produce sputum, sputum induction techniques can be used.
Sputum smear examination is a simple, rapid, feasible, and reliable method, though it has limited sensitivity. A positive result requires at least 5,000-10,000 bacilli per milliliter of sputum. In addition to the commonly used Ziehl-Neelsen staining method, the WHO currently recommends LED fluorescence microscopy for detecting acid-fast bacilli. This method is time-saving, convenient, and suitable for laboratories handling large numbers of samples. A positive sputum smear only indicates the presence of acid-fast bacilli and cannot differentiate between Mycobacterium tuberculosis and non-tuberculous mycobacteria. Further clinical data are needed to interpret its significance.
Mycobacterium tuberculosis culture provides accurate and reliable results, with sensitivity higher than smear examination. It is considered the gold standard for TB diagnosis. It also provides isolates for drug susceptibility testing and strain identification. The conventional Lowenstein-Jensen culture method takes 2-8 weeks. Liquid culture systems, such as the BACTEC-TB 960 method, can yield results within 10 days and improve isolation rates by 10%.
Patients with treatment failure, relapse, or retreatment should undergo drug susceptibility testing to diagnose drug-resistant cases, formulate appropriate chemotherapy regimens, and support epidemiological monitoring. The WHO considers the proportion method the gold standard for drug susceptibility testing. Newer methods, such as the BACTEC-TB 960 system, microscopic observation drug susceptibility testing, and phage amplification methods, have significantly shortened testing times and improved accuracy.
Molecular diagnostic techniques for TB use MTB-related genes as markers to detect MTB nucleic acids or drug resistance genes in samples. These techniques are highly sensitive, specific, and faster than conventional culture methods. A positive molecular test can serve as evidence of pathogen detection. Common methods include:
- Real-time fluorescent quantitative PCR (Xpert MTB/RIF): Detects the presence of MTB complex in clinical samples and its resistance to rifampicin.
- Isothermal amplification techniques: These include loop-mediated isothermal amplification (LAMP), cross-priming amplification (CPA), and simultaneous amplification and testing (SAT), which detect MTB complex in clinical samples under constant temperature conditions.
- High-throughput sequencing technologies, such as metagenomic next-generation sequencing (mNGS) and targeted next-generation sequencing (tNGS), do not rely on conventional microbial culture. They analyze microbial nucleic acids in clinical samples. While their diagnostic value for TB is still under evaluation, MTB-positive results require comprehensive interpretation alongside clinical and other test findings.
Electronic Bronchoscopy
Electronic bronchoscopy is commonly used for diagnosing bronchial TB and lymph node-bronchial fistulas. Bronchial TB may present as mucosal hyperemia, ulceration, erosion, tissue proliferation, scar formation, or bronchial stenosis. Biopsy samples can be taken from the lesion site for pathological examination and Mycobacterium tuberculosis culture. For pulmonary TB lesions, secretions or lavage fluid can be collected for pathogen testing, or transbronchial lung biopsy specimens can be obtained for examination.
Immunological Tests
Pulmonary TB is caused by infection with pathogenic microorganisms. Immunological techniques can be used to detect the immune response to mycobacteria, particularly Mycobacterium tuberculosis, to aid in differential diagnosis. However, due to the complexity of the immune response to Mycobacterium tuberculosis, immunological test results are only considered auxiliary indicators and cannot serve as definitive diagnostic or treatment evaluation criteria.
Tuberculin skin test (TST) is widely used to detect Mycobacterium tuberculosis infection but does not distinguish between infection and active disease. It is particularly useful for diagnosing TB in children, adolescents, and young adults. However, widespread BCG vaccination in many countries complicates interpretation, as a positive TST may reflect natural infection or vaccination. The WHO recommends purified protein derivative (PPD) or PPD-RT23 for the test.
A sufficient hypersensitivity reaction to Mycobacterium tuberculosis infection takes 4-8 weeks to develop. Before this, the TST may yield negative results. Malnutrition, HIV infection, measles, chickenpox, cancer, and severe bacterial infections, including severe TB (e.g., miliary TB or tuberculous meningitis), may result in negative or weakly positive TST results.
Interferon-gamma release assays (IGRAs) use specific antigens (ESAT-6 and CFP-10) to stimulate whole blood cells, measuring interferon-gamma levels or counting interferon-gamma-secreting T cells via enzyme-linked immunospot (ELISPOT) assays. IGRAs can distinguish Mycobacterium tuberculosis infection from BCG vaccination and most non-tuberculous mycobacterial infections, offering higher specificity than the TST. While IGRAs cannot confirm or exclude active TB, they can serve as supplementary diagnostic tools for bacteriologically negative cases, such as smear-negative pulmonary TB.
Diagnostic Workflow
Screening of Patients with Suspected Symptoms
The main suspicious symptoms of pulmonary TB include a cough or expectoration lasting more than two weeks, hemoptysis, afternoon low-grade fever, fatigue, diaphoresis, menstrual irregularities, or amenorrhea. A history of TB exposure or extrapulmonary TB should also be considered.
Determining Pulmonary TB
For patients with abnormal chest imaging findings, systematic examinations are required to determine whether the pulmonary lesions are of tuberculous or other origins. The diagnosis of TB relies on microbiological and pathological evidence. If a definitive diagnosis cannot be made immediately, a trial of empirical treatment for common pathogens for 10-14 days may be conducted. Most common infections will show changes on chest imaging, whereas TB lesions typically remain unchanged.
Assessing Disease Activity
If TB is diagnosed, it is necessary to determine whether the disease is active. Active TB lesions on chest imaging typically appear as poorly defined patchy opacities, sometimes with central liquefaction, cavities, or disseminated lesions. Calcified, nodular, or fibrotic lesions without bacterial shedding or symptoms are considered inactive TB.
Determining Bacterial Shedding
Confirming bacterial shedding is essential for identifying sources of infection.
Assessing Drug Resistance
Drug susceptibility testing is performed to determine whether the TB strain is drug-resistant.
Distinguishing New vs. Retreatment Cases
A detailed patient history is required to differentiate between new and retreatment cases, as their treatment regimens differ significantly.
Classification
Pulmonary tuberculosis (TB) can be classified based on different diagnostic methods.
Latent tuberculosis refers to individuals infected with Mycobacterium tuberculosis (MTB) but without clinical tuberculosis disease. There is no evidence of active TB based on clinical, bacteriological, or imaging findings.
Active tuberculosis refers to cases with clinical symptoms and signs related to TB, supported by evidence of active TB from microbiological, pathological, or imaging studies. Active TB can be further classified based on the location of the lesion, microbiological findings, drug resistance status, and treatment history.
Classification by Lesion Location
Pulmonary Tuberculosis
TB lesions occur in the lungs, trachea, bronchi, or pleura. Pulmonary TB is divided into the following five types based on lesion location:
- Primary pulmonary tuberculosis
- Hematogenous disseminated pulmonary tuberculosis
- Secondary pulmonary tuberculosis
- Tracheobronchial tuberculosis
- Extrapulmonary tuberculosis
Primary pulmonary tuberculosis includes primary complex and intrathoracic lymph node TB. This is commonly seen in children and adolescents, often asymptomatic or with mild symptoms, and usually associated with a history of TB exposure. Tuberculin skin tests are often strongly positive. Chest X-rays show the characteristic doughnut sign, which includes the primary lesion, lymphangitis, and hilar lymphadenopathy, forming the typical primary complex. Primary lesions typically resolve quickly without leaving any trace. If the X-ray only shows hilar lymphadenopathy, it is diagnosed as intrathoracic lymph node TB. Hilar lymph node TB can present as a tumor-like mass with clear margins and high density or as an inflammatory type with unclear margins and associated inflammatory infiltration.
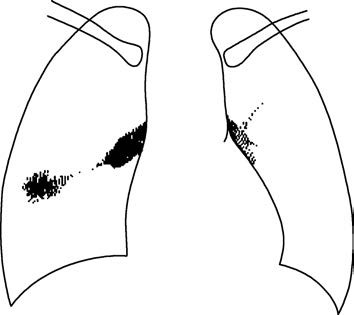
Figure 2 Schematic diagram of the primary complex
Hematogenous disseminated pulmonary tuberculosis includes acute, subacute, and chronic forms.
Acute hematogenous disseminated pulmonary tuberculosis (acute miliary tuberculosis) is common in infants, young children, and adolescents, especially those with malnutrition, infectious diseases, or long-term immunosuppressive therapy leading to significant immune suppression. It often coexists with primary pulmonary TB. Adults can also develop acute miliary TB, which presents with sudden onset, persistent high fever, and severe systemic symptoms. Generalized superficial lymphadenopathy, hepatosplenomegaly, and sometimes pale red miliary rashes on the skin may occur. Meningeal irritation signs, such as neck stiffness, and choroidal TB nodules in the fundus (in about 1/3 of cases) may also be observed. Imaging shows homogeneous, small, dense miliary opacities in both lungs.
Subacute and chronic hematogenous disseminated pulmonary tuberculosis has a slower onset, milder symptoms, and imaging findings of diffuse lesions in both lungs, predominantly in the upper and middle lobes. Lesions vary in size and density, with coexistence of fresh exudative lesions, old calcifications, and fibrosis.
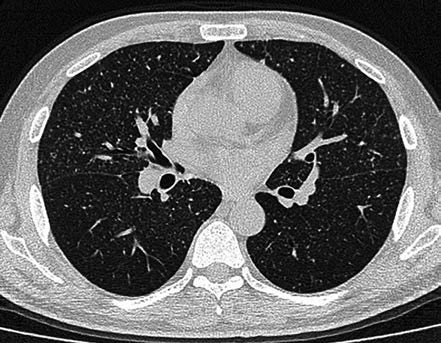
Figure 3 CT features of acute miliary pulmonary tuberculosis
Homogeneous millet-sized nodular opacities with consistent size and density in both lungs.
Secondary pulmonary tuberculosis is the most common type of TB in adults. Chest imaging reveals diverse manifestations, including infiltrative TB, caseous pneumonia, tuberculomas, chronic fibrocavitary TB, and destroyed lung.
Infiltrative pulmonary tuberculosis involves exudative and fibrous caseous proliferative lesions, often affecting the lung apex and subclavicular areas. Imaging shows small patchy or spotty opacities that may merge and form cavities. Exudative lesions tend to resolve quickly, while fibrous caseous proliferative lesions resolve slowly and may remain unchanged for a long time.
Figure 4 CT changes in infiltrative pulmonary tuberculosis
Exudative lesions in both upper lungs along the bronchial pathways, with visible cavity formation and smooth inner walls.
Cavitary lesions are irregular in shape, often formed by liquefaction of caseous exudative lesions, with indistinct cavity walls and multiple worm-eaten cavities. Tension cavities with thin walls may rapidly expand or contract due to valve-like bronchial obstruction. Cavitary infiltrative TB is often accompanied by bronchial dissemination, with clinical symptoms such as fever, cough, expectoration, and hemoptysis. Sputum frequently contains MTB. Effective therapy may result in sterilized cavities (non-closure cavities with negative sputum tests), where cavity walls are lined with fibrous tissue or epithelial cells. However, some cavities may retain caseous material, resulting in open-negative syndrome, requiring follow-up.
Caseous pneumonia occurs in patients with weakened immunity or high MTB load, often due to lymph node-bronchial fistulas or massive caseous material entering the lungs via the bronchi. Imaging shows lobar, ground-glass opacities with uniform density, later developing into worm-eaten cavities and disseminated lesions.
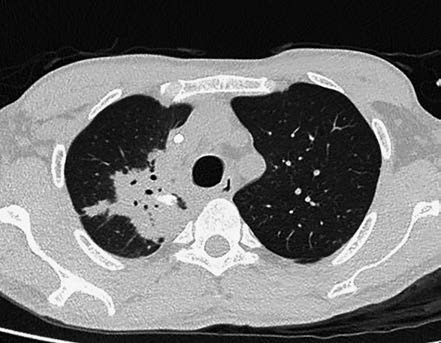
Figure 5 CT changes in caseous pneumonia
A large consolidation area in the right upper lung, showing air bronchograms and local calcified lesions.
Tuberculoma is formed by caseous lesions enveloped by fibrous membranes or obstructive healing of caseous cavities. Tuberculomas often contain calcifications or liquefied necrotic cavities, with over 80% showing satellite lesions, aiding in diagnosis. Diameter ranges from 2-4 cm, usually less than 3 cm.
Chronic fibrocavitary TB and destroyed lung involve long disease duration with repeated progression and severe pulmonary destruction. Imaging shows thick-walled fibrous cavities, extensive fibrosis, lung volume reduction, mediastinal and hilar displacement, chest wall collapse, pleural thickening, compensatory emphysema, and bronchial dissemination.
Tracheobronchial tuberculosis refers to TB affecting the mucosa and submucosa of the trachea and bronchi. It manifests as irregular thickening of the tracheal or bronchial walls, lumen narrowing or obstruction, and associated lung collapse, consolidation, bronchiectasis, or bronchial dissemination.
Tuberculous pleuritis includes dry pleuritis, exudative pleuritis, and tuberculous empyema.
Extrapulmonary Tuberculosis
Extrapulmonary tuberculosis refers to TB lesions occurring in organs or sites outside the lungs. It is named based on the affected organ or site.
Classification by Microbiological Findings
TB can be divided into microbiologically positive, microbiologically negative, and microbiologically untested TB. A positive result includes positive sputum smear, culture, or molecular biology tests.
Sputum test results are recorded as smear (+), smear (-), culture (+), culture (-), or molecular biology (+). If sputum is unavailable or untested, it is recorded as (no sputum) or (not tested).
Classification by Drug Resistance
TB can be classified into drug-sensitive TB and drug-resistant TB.
Drug-resistant TB is further divided into monoresistant TB, polyresistant TB, multidrug-resistant TB (MDR-TB), extensively drug-resistant TB (XDR-TB), and rifampicin-resistant TB (RR-TB).
Classification by Treatment History
New cases include the following:
- Patients who have not started anti-TB treatment.
- Patients undergoing standard therapy but have not completed the full course.
- Patients with irregular therapy lasting less than one month.
Retreatment cases include the following:
- Patients with treatment failure.
- Patients with recurrent positive sputum after completing a full course of treatment.
- Patients with unreasonable or irregular anti-TB treatment lasting ≥1 month.
Microbiologically negative pulmonary tuberculosis refers to pulmonary TB cases with negative microbiological test results, including smear, culture, and nucleic acid tests. Diagnosis requires exclusion of other non-TB pulmonary diseases and meeting the following criteria:
For pulmonary TB:
- Typical chest imaging findings of TB.
- Typical clinical manifestations of TB.
- Positive results in any of the following immunological tests: moderate or strong positive tuberculin skin test, positive interferon-gamma release assay (IGRA), or positive MTB antibody.
- Pathological confirmation of TB lesions in extrapulmonary tissues.
Diagnosis requires criteria 1 and any one of 2-4.
For tracheobronchial TB:
- Typical chest imaging findings.
- Bronchoscopic findings consistent with TB changes.
For tuberculous pleuritis:
- Typical chest imaging findings.
- Pleural effusion identified as exudative with elevated adenosine deaminase (ADA).
- Positive results in any of the following immunological tests: moderate or strong positive tuberculin skin test, positive IGRA, or positive MTB antibody.
Recording Format for Pulmonary Tuberculosis
TB is recorded based on its classification, lesion location, extent, sputum test results, and treatment history. For example:
- Primary pulmonary TB, right middle lung, smear (-), new case.
- Secondary pulmonary TB, bilateral upper lungs, smear (+), retreatment case.
- Hematogenous disseminated pulmonary TB (acute or chronic).
- Secondary pulmonary TB (e.g., infiltrative, chronic fibrocavitary).
- Complications (e.g., spontaneous pneumothorax, lung collapse), comorbidities (e.g., silicosis, diabetes), and surgeries (e.g., lobectomy, thoracoplasty) are recorded after the therapy history in sequence.
Differential Diagnosis
Infiltrative pulmonary tuberculosis should be differentiated from infectious pulmonary diseases such as bacterial pneumonia, pulmonary fungal infections, and pulmonary parasitic diseases. Detailed medical history, microbiological tests, or therapeutic response can help in further differentiation.
Tuberculoma should be distinguished from peripheral lung cancer, inflammatory pseudotumor, pulmonary hamartoma, and pulmonary sequestration.
- Peripheral lung cancer: Diagnosis is often confirmed through percutaneous lung biopsy or bronchoscopic lung biopsy with pathological examination.
- Inflammatory pseudotumor: This is an inflammatory granulomatous lesion of unknown etiology, often associated with a history of chronic pulmonary infection. Lesions may gradually shrink after anti-infective treatment.
- Pulmonary hamartoma: Typically presents as a solitary lesion with a "popcorn-like" appearance on imaging.
- Pulmonary sequestration: Common in young individuals and may remain asymptomatic for long periods if not accompanied by pulmonary infection. Lesions are usually located in the posterior basal segment of the lower lobes, more frequently on the left side. Contrast-enhanced CT may show separate blood supply.
Hematogenous disseminated pulmonary tuberculosis should be differentiated from bronchoalveolar carcinoma, pulmonary hemosiderosis, and diffuse interstitial lung diseases.
Bronchial lymph node tuberculosis should be differentiated from central lung cancer, lymphoma, and sarcoidosis. Diagnosis often requires pathological confirmation through bronchoscopy or endobronchial ultrasound-guided biopsy.
Pulmonary TB cavities should be differentiated from cancerous cavities, pulmonary cysts, and cystic bronchiectasis.
- Cancerous cavities: Typically have irregular walls with nodular protrusions inside the cavity, lack satellite lesions, and grow rapidly.
- Pulmonary cysts: Congenital abnormalities of lung tissue, often located in the upper lung fields. If infected, fluid levels may be visible within the cavity, but there are no satellite lesions. In the absence of infection, lesions may remain asymptomatic and unchanged for years.
- Cystic bronchiectasis: Commonly occurs in the middle and lower lung fields of both lungs. Patients often have a history of coughing up large amounts of purulent sputum or hemoptysis. High-resolution CT or bronchographic imaging with contrast can aid in diagnosis.
Treatment
Principles of Treatment
The principles of treatment for pulmonary tuberculosis are early initiation, regularity, completion of the full course, appropriate dosage, and combination therapy. The treatment regimen is divided into two phases: an intensive phase and a continuation phase.
Main Functions
Bactericidal Effect
Medications rapidly kill actively replicating Mycobacterium tuberculosis in lesions, converting patients from infectious to non-infectious, reducing tissue destruction, and resulting in rapid sputum smear conversion to negative.
Prevention of Drug-resistant Strains
Preventing the emergence of acquired drug-resistant mutant strains is crucial for successful treatment. The development of drug-resistant strains not only leads to treatment failure and relapse but also facilitates the spread of resistant bacteria.
Sterilization
The ultimate goal of treatment is to eradicate semi-dormant or metabolically slow Mycobacterium tuberculosis in lesions, ensuring no relapse or a very low relapse rate after completing the prescribed treatment course.
Biological Mechanisms
Effects of Drugs on Different Metabolic States and Locations of Mycobacterium Tuberculosis
Mycobacterium tuberculosis can be classified into four groups (A, B, C, D) based on metabolic states:
- Group A: Actively replicating bacteria, mainly located extracellularly in macrophages or caseous liquefied areas of lung cavities. This group constitutes the majority of Mycobacterium tuberculosis and is prone to developing drug-resistant mutants due to its large population.
- Group B: Semi-dormant bacteria located in acidic environments within macrophages or necrotic tissue of cavity walls.
- Group C: Semi-dormant bacteria with intermittent and brief growth spurts. Many biological characteristics of this group remain unclear.
- Group D: Dormant bacteria that do not replicate and are very few in number.
Different anti-tuberculosis drugs have varying effects on these groups:
- For Group A: Isoniazid > Streptomycin > Rifampicin > Ethambutol.
- For Group B: Pyrazinamide > Rifampicin > Isoniazid.
- For Group C: Rifampicin > Isoniazid.
Anti-tuberculosis drugs generally act on Group A. Isoniazid and rifampicin exhibit early bactericidal activity, rapidly reducing bacterial populations and infectiousness within 48 hours of treatment, leading to sputum smear conversion. This is critical for preventing acquired drug resistance. Groups B and C, being semi-dormant, are less responsive to anti-tuberculosis drugs and are referred to as persistent bacteria. Eradicating these groups helps prevent relapse. Group D bacteria are unaffected by anti-tuberculosis drugs.
Drug Resistance
Drug resistance arises from genetic mutations that reduce the efficacy of drugs against mutant strains. If a single drug is used during treatment, sensitive bacteria are killed, but naturally resistant mutant strains survive and proliferate, eventually replacing sensitive strains and becoming dominant. The larger the bacterial population in TB lesions, the more natural resistant mutants exist. Modern therapy often employs combination therapy to prevent resistance through cross-bactericidal effects. However, interrupted or irregular treatment with combination therapy can still lead to resistance. This is due to differences in the early bactericidal activity of various drugs, as well as variations in drug concentrations during bacterial regrowth or delayed growth. Therefore, even with combination therapy, treatment should not be interrupted, and directly observed therapy (DOT) is recommended for short-course regimens.
Intermittent Treatment
The theoretical basis for intermittent treatment lies in the delayed growth period of Mycobacterium tuberculosis. After exposure to isoniazid and rifampicin, the bacteria enter delayed growth periods lasting 6-9 days and 2-3 days, respectively. This delay allows for intermittent dosing. However, drugs like thiacetazone do not induce delayed growth periods and are unsuitable for intermittent use.
Single-dose Administration
A single daily dose of anti-tuberculosis drugs achieves higher peak blood concentrations compared to divided doses taken 2-3 times daily, with peak concentrations being approximately three times higher. Clinical studies have confirmed that single-dose administration is more effective than divided doses.
Common Anti-tuberculosis Drugs
Isoniazid is the most potent anti-tuberculosis drug, particularly in its early bactericidal activity. It is effective against both intracellular and extracellular Mycobacterium tuberculosis:
- Minimum inhibitory concentration (MIC): 0.025-0.05 μg/ml.
- Dosage: Adults: 300 mg daily (single dose); Children: 5-10 mg/kg daily, with a maximum dose of 300 mg/day. For tuberculous meningitis and disseminated pulmonary TB, higher doses may be used: Children: 20-30 mg/kg; Adults: 10-20 mg/kg.
- Adverse effects: Rare cases of drug-induced hepatitis. Use cautiously in patients with abnormal liver function, and monitor closely. Vitamin B6 (pyridoxine) can be used to prevent peripheral neuropathy.
Rifampicin (RFP, R):
- MIC: 0.06-0.25 μg/ml.
- Dosage: Adults: 8-10 mg/kg daily (450 mg for individuals ≤50 kg, 600 mg for those >50 kg); Children: 10-20 mg/kg daily. Intermittent dosing: 600-900 mg, 2-3 times per week.
- Adverse effects: Transient elevation in liver enzymes, flu-like symptoms, skin syndromes, and thrombocytopenia, particularly with intermittent therapy. Avoid during the first trimester of pregnancy and use cautiously thereafter.
Pyrazinamide (PZA, Z) has unique bactericidal activity against Group B bacteria in acidic environments:
- Dosage: Adults: 1.5 g/day; Children: 30-40 mg/kg daily.
- Adverse effects: Hyperuricemia, liver damage, anorexia, joint pain, and nausea.
Ethambutol (EMB, E):
- MIC: 0.95-7.5 μg/ml.
- Dosage: Adults: 0.75-1.0 g/day; Children: Not recommended due to difficulty in detecting visual side effects.
- Adverse effects: Optic neuritis; monitor vision and visual fields during treatment.
Streptomycin (SM, S) is effective against extracellular bacteria in alkaline environments:
- Dosage: 0.75 g/day (intramuscular injection), 5 times per week; Intermittent dosing: 0.75-1.0 g, 2-3 times per week.
- Adverse effects: Ototoxicity, vestibular dysfunction, and nephrotoxicity. Use cautiously in children, elderly, pregnant women, and individuals with hearing or renal impairment.
Fixed-dose combinations (FDCs) reduce the number of pills and improve patient adherence. They are primarily used in new cases of active pulmonary TB. Retreatment cases, tuberculous pleuritis, and extrapulmonary TB can also benefit from FDC regimens.
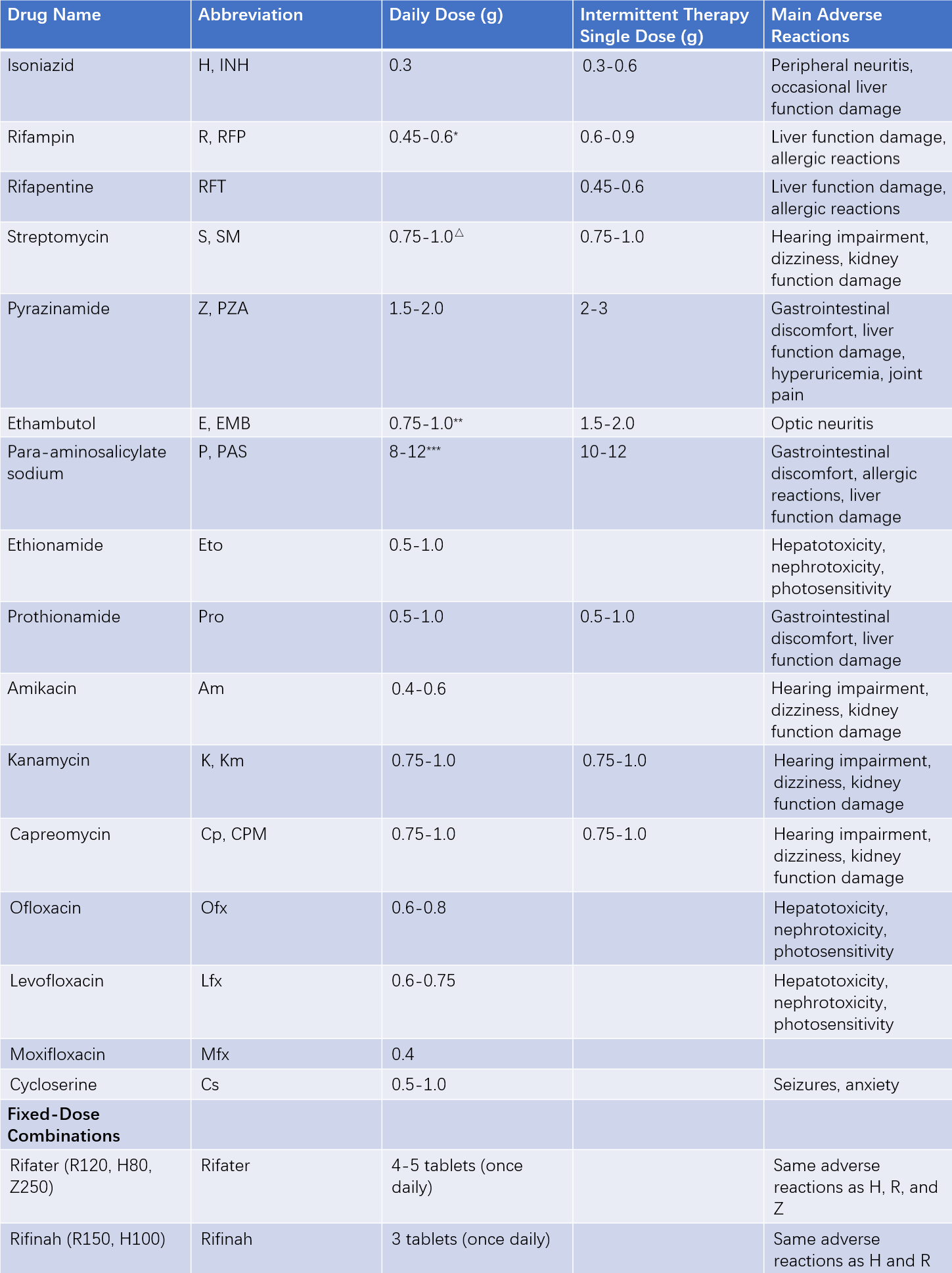
Table 1 Common anti-tuberculosis drugs for adults—Dosage and main adverse reactions
Notes:
- For body weight <50 kg, use 0.45 g; for >50 kg, use 0.6 g. Dosages of S, Z, and Th should also be adjusted according to weight.
△ Older patients: 0.75 g per dose.
** First 2 months: 25 mg/kg.
*** Administered twice daily (other drugs are taken once daily).
Standardized Treatment Regimens
To maximize the effectiveness of therapy in tuberculosis (TB) prevention and control, address issues such as misuse of anti-TB drugs, irrational or chaotic treatment regimens, poor treatment outcomes, high costs, overly short or prolonged treatment durations, drug supply problems, and resource wastage, standardized regimens have been developed. These regimens, proven effective through rigorous comparative studies, take into account factors such as efficacy, side effects, treatment costs, patient acceptance, and drug availability. Implementing standardized regimens has been shown to align with the cost-effectiveness principle.
Treatment Regimens for Newly Diagnosed Active Pulmonary Tuberculosis (both Smear-positive and Smear-negative)
2HRZE/4HR:
- Intensive phase: Isoniazid (H), Rifampicin (R), Pyrazinamide (Z), and Ethambutol (E), once daily for 2 months.
- Continuation phase: Isoniazid (H) and Rifampicin (R), once daily for 4 months.
2H3R3Z3E3/4H3R3:
- Intensive phase: Isoniazid (H), Rifampicin (R), Pyrazinamide (Z), and Ethambutol (E), three times a week or once every other day for 2 months.
- Continuation phase: Isoniazid (H) and Rifampicin (R), three times a week or once every other day for 4 months.
For newly diagnosed smear-positive patients whose sputum remains positive at the end of the 2-month intensive phase, the intensive phase should be extended by 1 month. The continuation phase regimen and duration remain unchanged. A sputum test should be repeated at the end of the third month. If sputum is negative at the end of the fifth month, the regimen is adjusted to 3HRZE/4HR or 3H3R3Z3E3. If sputum remains positive at the end of the fifth month or at the end of treatment, it is classified as treatment failure for new cases.
For newly diagnosed smear-negative patients, any positive sputum result during treatment is classified as treatment failure.
All patients with treatment failure should be re-registered, categorized as treatment failure, and treated with the retreatment regimen for smear-positive pulmonary TB.
WHO recommendations:
- For drug-sensitive pulmonary TB patients aged ≥12 years, a 4-month short-course regimen (2HPMZ/2HPM) can be used. Here, H = Isoniazid, P = Rifapentine, M = Moxifloxacin, Z = Pyrazinamide.
- For children and adolescents aged 3 months to 16 years with non-severe TB, a 4-month short-course regimen (2HRZ(E)/2HR) can be used. Non-severe TB includes peripheral lymph node TB, intrathoracic lymph node TB without airway obstruction, simple tuberculous pleuritis, or limited non-miliary, non-cavitary, paucibacillary pulmonary TB confined to one lobe.
Although the 2022 WHO guidelines recommend new 4-month short-course regimens, the 6-month regimen (2HRZE/4HR) remains a conservative and reliable option. Further research is needed to evaluate the applicability of the new regimens to the TB population.
Retreatment of Pulmonary Tuberculosis
Pulmonary TB patients requiring retreatment include those with a history of unreasonable or irregular anti-TB treatment for ≥1 month, as well as those with treatment failure or relapse. According to the WHO 2017 guidelines, there is no longer a fixed standard retreatment regimen. All retreatment patients should undergo drug susceptibility testing, and individualized treatment regimens should be developed based on resistance results. Retreatment TB is classified into two categories: rifampicin-sensitive TB and rifampicin-resistant TB.
For rifampicin-sensitive or resistance-unknown TB patients, the first choice is to follow the standardized treatment regimen for new cases.
Drug-resistant patients should be treated with regimens for drug-resistant TB.
Treatment of MDR-TB or RR-TB
Drug-resistant TB, especially MDR-TB (multi-drug-resistant TB, resistant to at least isoniazid and rifampicin) and RR-TB (rifampicin-resistant TB), poses a significant challenge to global TB control. Pharmacotherapy remains the primary treatment method for drug-resistant TB, and the development of standardized regimens is crucial for ensuring treatment success.
Types of Anti-TB drugs and Dosages
Drugs Used in Long-course Regimens
According to WHO recommendations, anti-TB drugs used in MDR/RR-TB long-course regimens are categorized into three groups (A, B, C) based on priority.
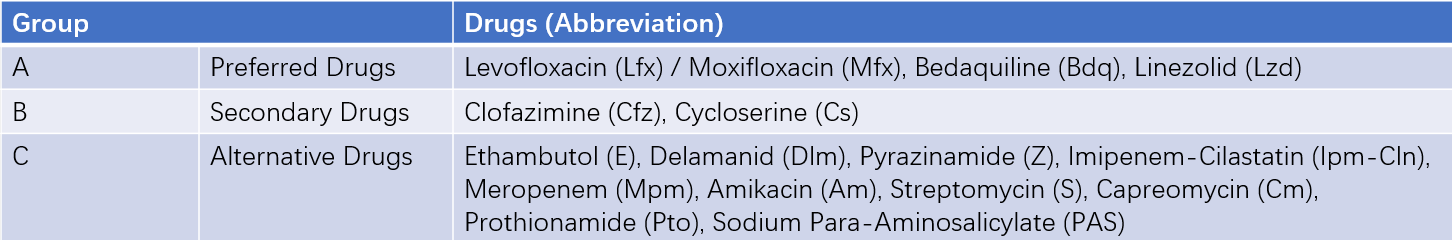
Table 2 Long-term treatment regimens for rifampin-resistant tuberculosis
Drugs Used in Short-course Regimens
Anti-TB drugs used in short-course regimens include:
- Moxifloxacin (Mfx)
- Clofazimine (Cfz)
- Ethambutol (E)
- Pyrazinamide (Z)
- High-dose Isoniazid (H)
- Prothionamide (Pto)
- Amikacin (Am)
Treatment Regimens
Treatment regimens are divided into long-course and short-course regimens. If a patient is eligible for a short-course regimen, it should be prioritized.
Long-course Regimen
A long-course regimen consists of at least four effective anti-TB drugs and lasts 18-20 months. It can be standardized or individualized.
Principles for developing regimens:
- Include all Group A drugs and at least one Group B drug.
- If only 1-2 Group A drugs are available, include all Group B drugs.
- If Group A and B drugs cannot form a regimen, Group C drugs may be added.
Recommended standardized regimens:
- For fluoroquinolone-sensitive cases: 6Lfx (Mfx) Bdq Lzd (Cs) Cfz / 12Lfx (Mfx) Cfz Lzd (Cs).
- For fluoroquinolone-resistant cases: 6Bdq Lzd Cfz Cs / 14Lzd Cfz Cs.
Short-course Regimen
A short-course MDR-TB regimen lasts 9-12 months and is mostly standardized.
Recommended regimen: 4-6 Am Mfx Pto Cfz Z H (high-dose) E / 5 Mfx Cfz ZE.
Treatment phases:
- Intensive phase: If sputum culture remains positive at the end of 4 months, the intensive phase can be extended to 6 months.
- Continuation phase: If sputum culture remains positive at the end of 6 months, the treatment is classified as a failure, and the patient should switch to an individualized regimen.
Eligibility criteria:
- Patients who have not received or have received second-line drugs in the short-course regimen for less than 1 month.
- Patients sensitive to fluoroquinolones and second-line injectable drugs.
Exclusions:
- Patients who cannot tolerate or are at risk of toxicity from any drug in the short-course regimen (e.g., drug interactions).
- Pregnant patients.
- Patients with disseminated TB, TB meningitis or central nervous system TB, or extrapulmonary TB with HIV co-infection.
Other Treatments
Symptomatic Treatment
The general symptoms of pulmonary tuberculosis (TB) usually improve or disappear quickly under rational therapy, and no special treatment is needed. Hemoptysis (coughing up blood) is a common symptom of pulmonary TB. For minor hemoptysis, reassurance, reducing patient anxiety, bed rest, and hemostatic medications such as aminocaproic acid, tranexamic acid, etamsylate, and carbazochrome can be used. For massive hemoptysis, vasopressin can be administered to stop bleeding. Vasopressin works by constricting small arteries, reducing pulmonary circulation and achieving hemostasis. However, it is contraindicated in patients with hypertension, coronary atherosclerotic heart disease, heart failure, and pregnant women. During vasopressin use, adverse reactions such as hyponatremia, hypokalemia, diarrhea, and hypertension should be monitored. For massive hemoptysis caused by bronchial artery damage, bronchial artery embolization may be considered. If a patient with massive hemoptysis suddenly stops coughing up blood and develops symptoms such as shortness of breath, pallor, cyanosis of the lips, and restlessness, it may indicate suffocation due to hemoptysis, requiring immediate rescue.
Glucocorticoids
The use of glucocorticoids in TB treatment is primarily for their anti-inflammatory effects. They are only used in cases of severe tuberculous symptoms and must be administered alongside effective anti-TB drugs. The dosage depends on the condition, typically 20 mg of prednisone taken orally once daily for 1-2 weeks, followed by a weekly reduction of 5 mg, with a total treatment duration of 4-8 weeks.
Immunotherapy
Immunotherapy for TB involves using immunomodulators to regulate the immune system, enabling an appropriate immune response to prevent and treat the disease. Commonly used immunotherapies and immunomodulators include injectable Mycobacterium bovis, cytokines (e.g., IL-2, γ-interferon), and thymic extracts (e.g., thymosin or thymopentin).
Surgical Treatment
Surgical treatment for pulmonary TB is currently indicated in cases where rational therapy is ineffective, including:
- Multidrug-resistant thick-walled cavities
- Large caseous lesions
- Tuberculous empyema
- Bronchopleural fistulas
- Massive hemoptysis unresponsive to conservative treatment
Pulmonary Tuberculosis and Related Diseases
HIV/AIDS
TB is the most common opportunistic infection in HIV/AIDS patients. HIV/AIDS accelerates the progression and infection of latent TB and is the most significant risk factor for TB development. The two conditions exacerbate each other, leading to a loss of the body's defense mechanisms, rapid disease progression, and a high mortality rate.
Hepatitis
Isoniazid, rifampicin, and pyrazinamide all have potential hepatotoxicity. Liver function should be monitored regularly before and during treatment. In regions with endemic infectious hepatitis, determining the cause of hepatitis can be challenging. If hepatitis is severe but TB treatment is necessary, the 2SHE/10HE regimen can be considered.
Diabetes
The incidence of diabetes combined with pulmonary TB is increasing annually. The two diseases mutually influence each other, with diabetes significantly impairing the effectiveness of TB treatment. Effective TB treatment requires strict diabetes control.
Silicosis
Patients with silicosis are at high risk of developing pulmonary TB. Over 50% of patients with stage III silicosis develop pulmonary TB. Diagnosing TB in silicosis patients emphasizes repeated sputum examinations, particularly using culture methods.
COVID-19
Patients with both TB and COVID-19 face a higher risk of mortality. Similarly, individuals with HIV or TB/HIV co-infection are at increased risk of severe COVID-19 and have higher case fatality rates. Other major health risk factors for poor outcomes in both COVID-19 and TB include diabetes and smoking.
Control Strategies and Measures
Directly Observed Treatment (DOT)
DOT ensures that every dose of medication is taken under the direct supervision of healthcare personnel or trained family supervisors. If a dose is missed, remedial measures must be taken to ensure adherence to prescribed therapy. DOT improves treatment compliance and cure rates while reducing the incidence of multidrug-resistant TB cases.
Case Reporting and Referral
All levels of medical and preventive institutions must assign dedicated personnel to ensure timely, accurate, and complete reporting of TB cases. Proper referral processes must also be established.
Case Registration and Management
Due to the chronic nature, high relapse rate, and infectiousness of pulmonary TB, long-term follow-up is essential to monitor the patient's progress from onset, through treatment, to cure. Case registration allows for better epidemic monitoring and management.
BCG Vaccination
BCG vaccination is widely recognized as ineffective in preventing adult pulmonary TB but is effective in preventing tuberculous meningitis and miliary TB in children. After neonatal BCG vaccination, isolation from TB patients must still be maintained.
Prophylaxis
Prophylaxis for individuals with latent TB infection (LTBI) reduces the risk of developing active TB and is an important measure. Candidates for preventive treatment include:
- Children under 5 years old with LTBI who have had close contact with smear-positive TB patients.
- HIV-infected individuals and AIDS patients with LTBI, or individuals deemed necessary for treatment by clinicians despite negative infection tests.
- Recently infected individuals, such as students with LTBI who have had close contact with active TB patients.
- Other high-risk groups: Those undergoing tumor necrosis factor therapy, long-term dialysis, or preparing for organ or bone marrow transplantation; patients with silicosis; and individuals with LTBI who are on long-term corticosteroids or other immunosuppressive treatments.
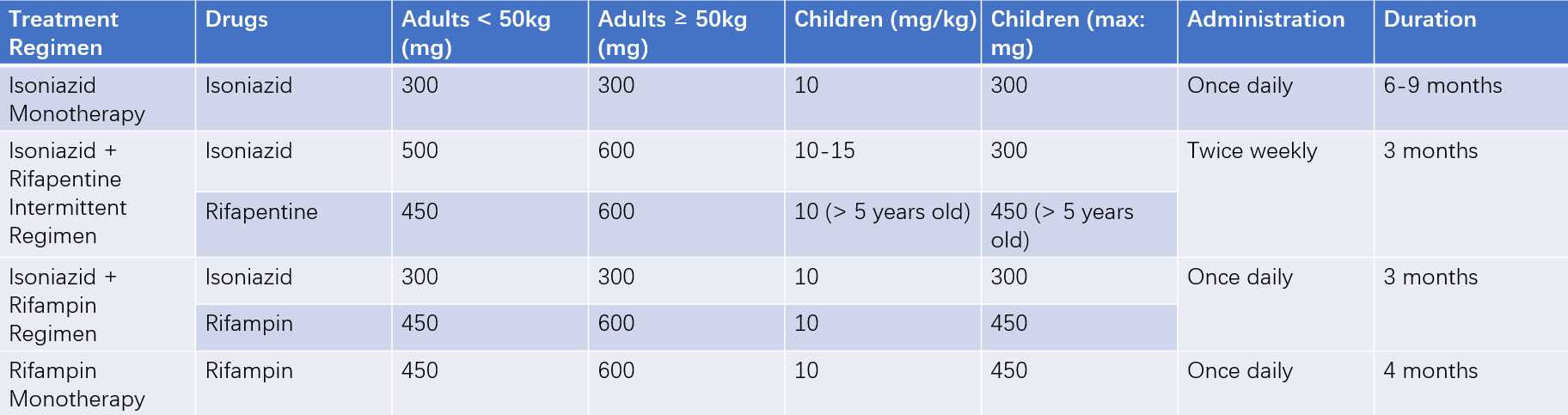
Table 3 Preventive treatment regimens for tuberculosis