Anatomical Structure of the Hypothalamus
Anatomy of the Hypothalamus
The hypothalamus is a wedge-shaped, small, gray matter-dominant structure located in the lower part of the diencephalon. It is situated below the thalamus, which appears as a protrusion on the posterior wall of the third ventricle in the sagittal section of the brain. Extending downward, the hypothalamus connects to the pituitary stalk. On the ventral surface of the brain, the hypothalamus is identifiable as a prominent elevation, flanked posteriorly by paired mammillary bodies and centrally by the tuberal eminence. The hypothalamus weighs approximately 4 grams in adults, accounting for less than 1% of the total brain weight. It contains many neural nuclei and is connected to the brain and brainstem through afferent and efferent nerve fibers.
The hypothalamus is anatomically divided into four regions, extending from anterior to posterior: the preoptic region, supraoptic region, tuberal region, and mammillary region. Each region is further divided into three longitudinal zones from the third ventricle outward: the periventricular zone, the medial zone, and the lateral zone.
Connection with the Pituitary
The hypothalamus is closely linked to the pituitary gland, with its median eminence extending downward to connect with the pituitary stalk. This represents the most proximal anatomical and functional relationship between the two, serving as a critical regulatory region for pituitary function and the common pathway for hormone secretion into the pituitary.
The hypothalamus and the neurohypophysis (posterior pituitary) are connected by neural fibers. Axons originating from the supraoptic nucleus and paraventricular nucleus form the supraoptic-paraventricular hypothalamo-hypophyseal tract, while neural fibers from the middle and posterior hypothalamus form the tuberohypophyseal tract. These neural fibers descend through the pituitary stalk to enter the neurohypophysis, where neurohormones are stored. The neurohypophysis is essentially a continuation of the hypothalamus.
In contrast, the hypothalamus and adenohypophysis (anterior pituitary) are connected via a neurovascular interface. Hypothalamic nerve axons project into the primary capillary plexus of the hypophyseal portal system at the median eminence and pituitary stalk, where releasing hormones are secreted into the bloodstream and subsequently delivered to the adenohypophysis. There, these hormones stimulate or inhibit the secretion of pituitary hormones. Different regions of the hypothalamus regulate different parts of the portal circulation through distinct vascular drainage pathways.
Functional Roles of the Hypothalamus
Functional Zonation of Hypothalamic Neural Nuclei
Different regions and nuclei within the hypothalamus exhibit distinct functions and morphologies.
Hypothalamus-Pituitary-Gonadal Axis Regulation
The anterior portion of the hypothalamus is associated with gonadotropin secretion. Estrogen exerts a positive feedback effect in this region, leading to the mid-cycle release of gonadotropins, particularly luteinizing hormone (LH), to trigger ovulation.
The middle and posterior portions of the hypothalamus contribute to gonadotropin secretion and are influenced by estrogens through negative feedback, which regulates the tonic secretion of gonadotropins.
The paraventricular nucleus and arcuate nucleus in the anterior ventral hypothalamus express the KISS1 gene, whose product, kisspeptin, plays a key role in initiating puberty and regulating the hypothalamus-pituitary-gonadal axis. Its target cells are gonadotropin-releasing hormone (GnRH) neurons in the median eminence.
Hypothalamus-Pituitary-Thyroid Axis Regulation
The anterior hypothalamus is associated with thyroid-stimulating hormone (TSH) secretion. The paraventricular nucleus in the anterior ventral region contains numerous thyrotropin-releasing hormone (TRH)-producing cells, which regulate TSH secretion.
Hypothalamus-Pituitary-Adrenal Axis Regulation
The regulation of adrenocorticotropic hormone (ACTH) secretion occurs across broad areas of the hypothalamus. As a result, hypothalamic lesions rarely lead to a significant reduction in corticotropin-releasing hormone (CRH) concentrations within the median eminence.
Regulation of Water and Salt Metabolism
The median preoptic nucleus, located at the anterior edge of the third ventricle, is one of the osmosensitive regions within the hypothalamus. The paraventricular nucleus, situated in the periventricular zone, contains neurons that secrete oxytocin and/or antidiuretic hormone (ADH). Similarly, the supraoptic nucleus, located lateral to the optic chiasm and optic tracts, secretes ADH. Several osmosensitive regions near the hypothalamus are capable of detecting even 1% changes in plasma osmolality, modulating oxytocin and ADH secretion accordingly.
Regulation of Circadian Rhythms
The suprachiasmatic nucleus, positioned above the optic chiasm, works in conjunction with the sleep centers in the anterior hypothalamus and the wakefulness centers in the posterior hypothalamus to regulate sleep-wake cycles and circadian rhythms.
Appetite Center
The ventromedial nucleus serves as the satiety center, while the lateral hypothalamic area functions as the feeding center. Both play critical roles in the regulation of appetite.
Thermoregulatory Center
The preoptic area of the anterior hypothalamus and the posterior hypothalamus are involved in the regulation of body temperature.
Functions of Hypothalamic Neurosecretory Cells
Hypothalamic neurosecretory cells possess both neuronal and endocrine gland cell traits. These cells perform several key functions:
- Neurotransmitter Function: Facilitate synaptic communication.
- Neuromodulator Function: Influence the excitability and responsiveness of other neurons.
- Signal Integration Function: Integrate multiple signals into a unified output, mediated via specific neurohormones that act on other nerve cells or target cells.
- Target Cell Function: Exhibit receptors for various hormones on membranes or within the cell, enabling them to respond to circulating or paracrine hormones with corresponding hormone secretion.
Hypothalamic neurosecretory cells are morphologically classified into the following categories:
Large Neurosecretory Cells
These cells are relatively large and are located in the supraoptic nucleus and paraventricular nucleus of the hypothalamus. Their axons form the supraoptic-paraventricular hypothalamo-hypophyseal tract, with axonal terminals ending primarily in the neurohypophysis (posterior pituitary) and partially in the median eminence. The supraoptic nucleus predominantly produces antidiuretic hormone (ADH), while the paraventricular nucleus primarily synthesizes oxytocin. However, both nuclei are also capable of producing a variety of other hormones. Neurohormones synthesized in these cells are transported along axons and stored in the neurohypophysis.
Small Neurosecretory Cells
These cells produce various regulatory neuropeptides that modulate pituitary hormones, including releasing hormones and release-inhibiting hormones. Their axonal fibers originate from the arcuate nucleus (infundibular nucleus), ventromedial nucleus, periventricular nucleus, and suprachiasmatic nucleus at the base of the hypothalamus and terminate in the median eminence.
Other Cells
Glial cells in the median eminence of the hypothalamus are known as tanycytes, while specialized differentiated astrocytes in the neurohypophysis are called pituicytes. Tanycytes and pituicytes are in close association with blood vessels. These cells regulate hypothalamic hormone release by modulating the "opening" and "closing" of surrounding blood vessels. Tanycytes and pituicytes possess blood-brain barrier functions. Additionally, pituicytes express receptors for opioids, ADH, and β-adrenergic agonists, making them sensitive to various hormones or neurotransmitters. Tanycytes may also regulate the substance transport in the portal system of the pituitary by interacting with hypothalamic nerve axons.
Hypothalamic Endocrine Functions and Regulation
Hormones Secreted by the Hypothalamus
In addition to synthesizing and secreting regulatory peptides such as gonadotropin-releasing hormone (GnRH), growth hormone-releasing hormone (GHRH), somatostatin (SS), thyrotropin-releasing hormone (TRH), corticotropin-releasing hormone (CRH), melanocyte-stimulating hormone-releasing factor (MRF), prolactin-inhibiting factor (PIF), ADH, and oxytocin, the hypothalamus also releases numerous neurotransmitters, neuromodulators, cytokines, growth factors, excitatory amino acids, and nitric oxide (NO). Moreover, hypothalamic neurosecretory cells express a variety of hormone receptors, allowing them to respond to paracrine/autocrine signals, pituitary hormones, circulating hormones, and metabolic byproducts, thereby enabling feedback regulation.
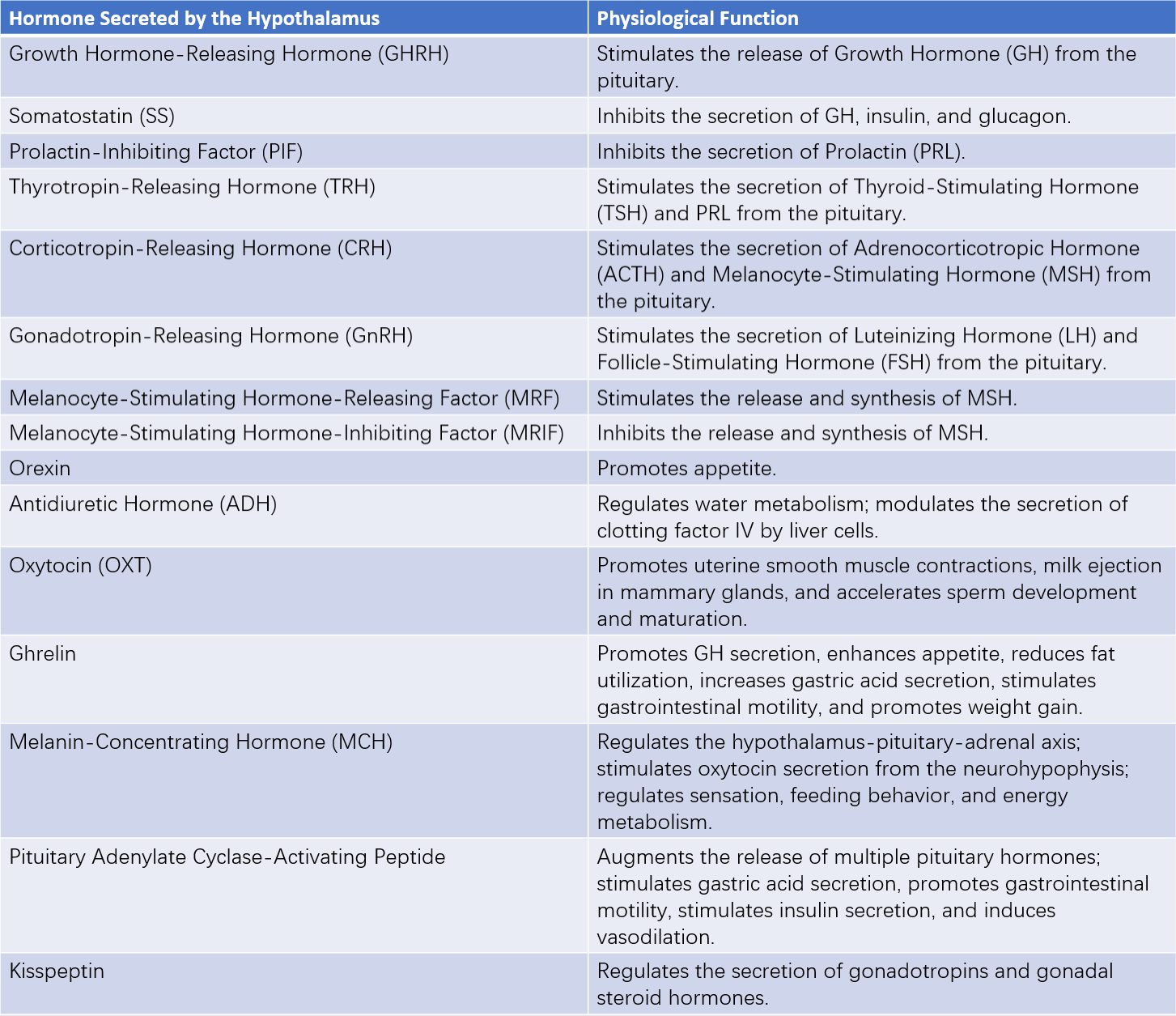
Table 1 Hormones secreted by the hypothalamus and their main physiological functions
Note:
GH: Growth Hormone
TSH: Thyroid-Stimulating Hormone
PRL: Prolactin
ACTH: Adrenocorticotropic Hormone
MSH: Melanocyte-Stimulating Hormone
LH: Luteinizing Hormone
FSH: Follicle-Stimulating Hormone
The relationship between hypothalamic hormones and pituitary hormones is typically one-to-one, with stimulatory hormones from the hypothalamus corresponding to specific target hormones from the pituitary. Exceptions exist, such as TRH, which stimulates the secretion of both TSH and prolactin (PRL); GnRH, which promotes the release of both LH and FSH; and somatostatin, which suppresses the release of GH and other hormones.
Feedback Regulation by Target Gland Hormones
Hormones from endocrine glands provide feedback regulation to the hypothalamus-pituitary axis (long-loop negative feedback), with the site of regulation varying by hormone. For example, adrenal corticosteroids and sex hormones primarily regulate the hypothalamus, while thyroid hormones mainly act on the pituitary. Additionally, target gland hormones can influence higher neural centers above the hypothalamus. The feedback regulation by target gland hormones is generally negative; increased hormone levels in the blood inhibit the secretion of corresponding hypothalamic-pituitary hormones.
Sex hormones exhibit complex effects. In the hypothalamic arcuate nucleus, which regulates tonic secretion of gonadotropins, estradiol and progesterone inhibit GnRH release (negative feedback). During the mid-menstrual cycle, the abrupt secretion of gonadotropins associated with ovulation is regulated by neurons in the anterior hypothalamus and supraoptic region. Sex hormones exert a stimulatory effect on GnRH release in this region (positive feedback).
Pituitary hormones also exert negative feedback on the hypothalamic releasing hormones (short-loop negative feedback). Such regulation occurs through two mechanisms: one via systemic blood circulation to the hypothalamus, and another potentially through reverse blood flow in the portal venous system or periportal spaces to the hypothalamus.
Regulation by the Nervous System, Neurotransmitters, and Cytokines
The nervous system plays a critical role in modulating the hypothalamus-pituitary-target gland axis. Sensory inputs such as light, sound, and smell are known to influence gonadal activity in mammals. Stress stimuli, such as surgery or trauma, activate peripheral afferent nerves to stimulate pituitary secretion of adrenal corticosteroids. These sensory signals affect hypothalamic endocrine functions via the midbrain reticular formation and the limbic system (limbic-midbrain loop). Higher neurological activity, such as psychological tension and anxiety, can also enhance hypothalamus-pituitary-adrenal cortex activity (stress response).
Recent studies indicate that peptide neurotransmitters (e.g., opioid peptides) significantly impact hypothalamic hormone release. For instance, administration of endorphins or β-endorphins to humans suppresses CRH and GnRH secretion while stimulating the release of TRH and GHRH. Monoaminergic neurotransmitters directly synapse onto hypothalamic peptidergic neurons, regulating their activity.
Cytokines also exert apparent autocrine, paracrine, and endocrine regulatory roles. For example, interleukin-1 (IL-1) inhibits the secretion of GnRH and TRH in the hypothalamus while stimulating dopamine release. Tumor necrosis factor-alpha (TNF-α) may promote the secretion of GnRH.