Pulmonary thromboembolism (PTE) is the most common type of pulmonary embolism. It is caused by thrombi originating from the venous system or the right side of the heart, which obstruct the pulmonary arteries or their branches. PTE is characterized by clinical and pathophysiological features of dysfunctional pulmonary circulation and respiratory system. The primary source of thrombi in PTE is deep venous thrombosis (DVT). Essentially, DVT and PTE represent different manifestations of the same disease process at different locations and stages, collectively referred to as venous thromboembolism (VTE).
Recurrent thromboembolism in the pulmonary arteries, along with unresolved and organized thrombi following embolism, leads to chronic mechanical obstruction of the blood vessels. This condition is termed chronic thromboembolic pulmonary disease (CTEPD). As the disease progresses, pulmonary vascular remodeling occurs, causing further narrowing or occlusion of the vessels, increased pulmonary vascular resistance (PVR), and progressively elevated pulmonary arterial pressure. Over months or years, this can eventually result in right ventricular hypertrophy and right heart failure, a condition known as chronic thromboembolic pulmonary hypertension (CTEPH).
Epidemiology
The incidence of VTE increases with age, with patients over 40 years old being at significantly higher risk. The risk approximately doubles every 10 years.
PTE has a high mortality and disability rate. An international registry study reported that the 7-day and 30-day all-cause mortality rates for PTE were 1.9%-2.9% and 4.9%-6.6%, respectively.
Risk Factors
Any factor that leads to venous stasis, vascular endothelial injury, or hypercoagulable states (Virchow's triad) is a risk factor for VTE. These can be categorized into hereditary and acquired factors.
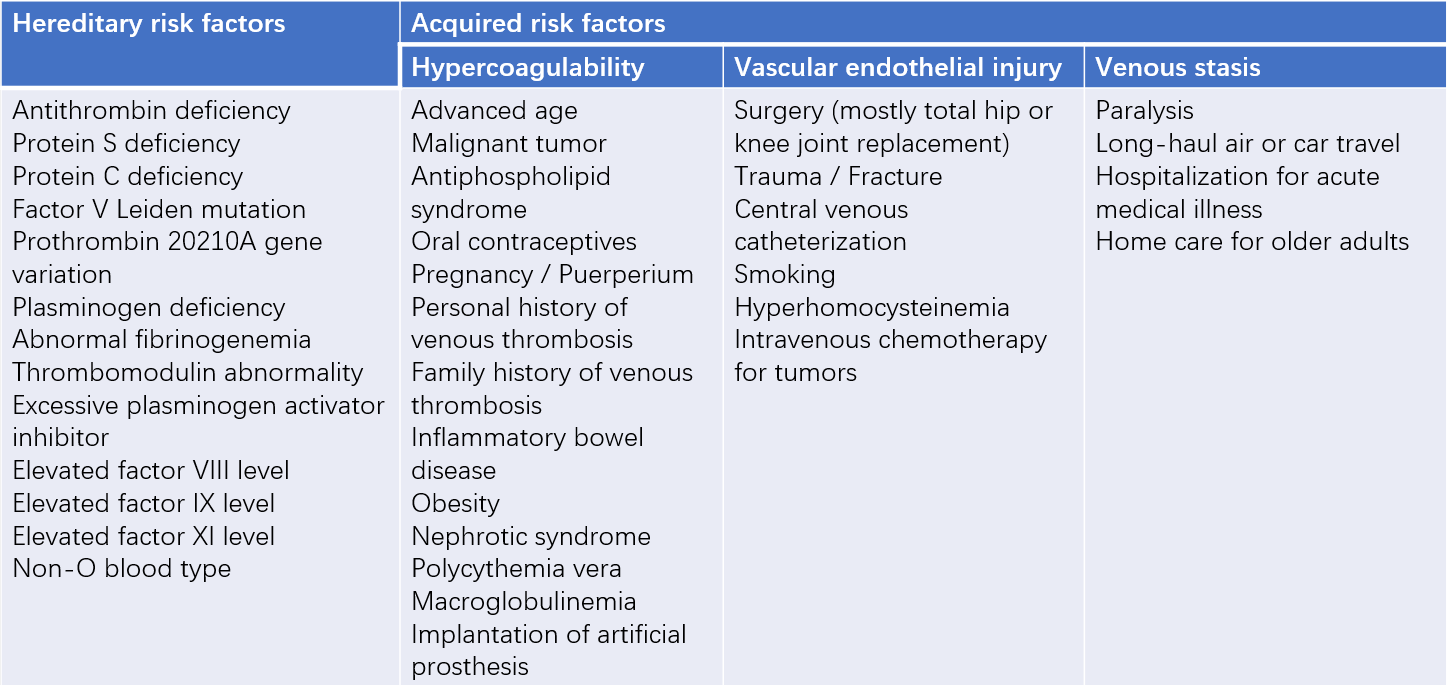
Table 1 Common risk factors for venous thromboembolism (VTE)
Hereditary Risk Factors
These are caused by genetic variations and are often characterized by recurrent arterial or venous thrombosis as the main clinical manifestation. Patients under 50 years of age who experience recurrent VTE without obvious triggers or have a family history of VTE should be evaluated for thrombophilia.
Acquired Risk Factors
These are pathological or physiological abnormalities acquired later in life that predispose to VTE, often transient or reversible. Malignancy is a major risk factor for VTE, with the risk significantly increasing during active cancer. VTE shares some common risk factors with arterial diseases, such as smoking, obesity, hypercholesterolemia, hypertension, and diabetes. Myocardial infarction and heart failure can also increase the risk of VTE. Age is an independent risk factor, with the incidence of VTE rising steadily with advancing age.
In some patients, no clear risk factors can be identified even after thorough evaluation; this is referred to as idiopathic VTE. Some cases of idiopathic VTE may be associated with occult malignancies, warranting careful screening and follow-up.
Pathology and Pathophysiology
The thrombi in pulmonary thromboembolism (PTE) can originate from the inferior vena cava, superior vena cava, or the right heart chambers, with the majority arising from the deep veins of the lower extremities. Approximately 70% of PTE patients have deep vein thrombosis (DVT) in the lower limbs, and about 50% of patients with proximal DVT have symptomatic PTE.
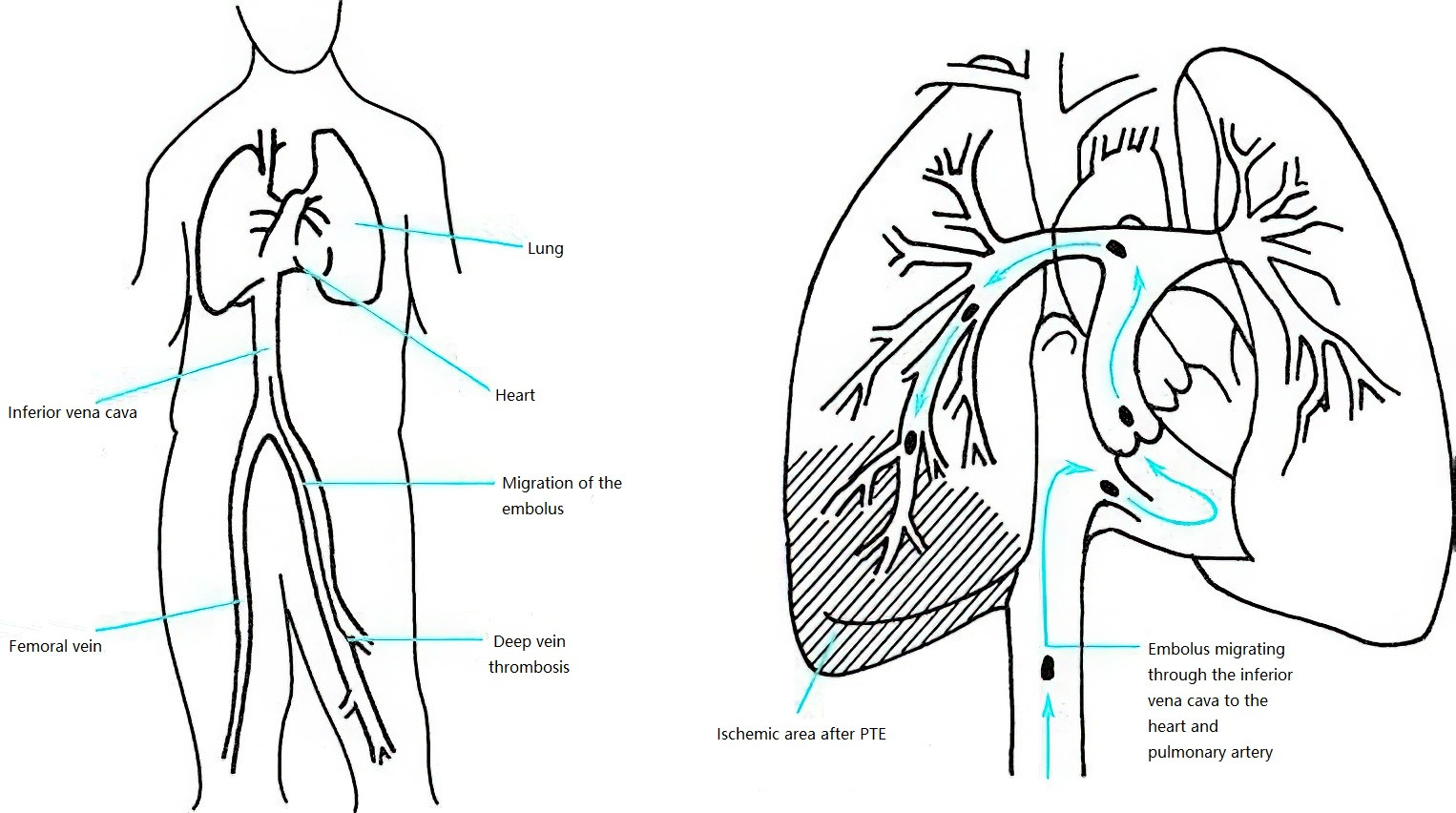
Figure 1 Mechanism of pulmonary thromboembolism (PTE) formation
After forming in the peripheral deep veins, thrombi detach and travel with venous blood flow to the pulmonary arteries, resulting in pulmonary arterial thromboembolism.
Increased Pulmonary Vascular Resistance (PVR) and Cardiac Dysfunction
When emboli obstruct the pulmonary arteries and their branches to a significant extent (30%-50%), mechanical obstruction and other factors lead to increased PVR, which subsequently increases the afterload of the right ventricle and raises pulmonary arterial pressure. Right ventricular dilation causes the interventricular septum to shift toward the left, impairing left ventricular function. This results in impaired early diastolic filling of the left ventricle, reduced cardiac output, systemic hypotension, and hemodynamic instability. Reduced cardiac output, combined with systemic hypotension and elevated right ventricular pressure, decreases coronary perfusion pressure, particularly affecting the subendocardial myocardium of the right ventricle, leading to hypoperfusion.
Respiratory Dysfunction
Reduced cardiac output leads to decreased mixed venous oxygen saturation. PTE causes vascular obstruction, reducing blood flow to the embolized regions of the lung and increasing dead space ventilation. Redistribution of pulmonary blood flow to non-obstructed areas leads to ventilation-perfusion mismatch and hypoxemia. Approximately 1/3 of patients develop reopening of the foramen ovale due to increased right atrial pressure, resulting in right-to-left shunting, which can cause severe hypoxemia (and increase the risk of paradoxical embolism and sudden death). Distal small emboli may cause localized hemorrhagic atelectasis, known as pulmonary infarction, accompanied by alveolar hemorrhage, which may manifest as hemoptysis, pleuritis, and pleural effusion, further impairing gas exchange.
Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
In some cases, acute PTE thrombi fail to dissolve completely after treatment. Thrombus organization, chronic inflammation, and thickening of the pulmonary arterial intima can lead to chronic PTE. Recurrent embolization of the pulmonary arteries due to repeated DVT episodes is another major cause of chronic PTE. Thrombus organization is accompanied by varying degrees of vascular remodeling and in situ thrombosis, resulting in lumen narrowing or occlusion, progressively increasing PVR and pulmonary arterial pressure, ultimately leading to pulmonary hypertension. Factors such as hypoxemia can exacerbate this process, further increasing the right ventricular afterload and eventually causing right heart failure.
Clinical Manifestations
Acute PTE lacks specific clinical manifestations and varies widely in severity. Mild cases may be asymptomatic, while severe cases can present with hemodynamic instability, shock, or even sudden death. The presence of DVT symptoms should also be considered during diagnosis.
Symptoms
Dyspnea and shortness of breath are the most common symptoms, often exacerbated by physical activity and relieved or absent at rest. Patients may report sudden chest tightness during positional changes, bowel movements, or stair climbing.
Chest pain includes pleuritic chest pain and angina-like pain. The former is more common and is characterized by pain that worsens significantly with deep breathing or coughing. The latter, resembling severe substernal squeezing pain, occurs in a minority of patients and mimics angina attacks.
Hemoptysis occurs in approximately 1/3 of patients, usually within 24 hours after pulmonary infarction. It is often mild, with massive hemoptysis being rare.
The mechanism of agitation, anxiety, and a sense of impending doom is unclear but may be related to chest pain or hypoxemia.
Cough can be seen in about 1/3 of patients, typically presenting as a dry cough or cough with scant white sputum.
Syncope may be the sole or initial symptom of acute PTE. It is primarily caused by large emboli obstructing more than 50% of the pulmonary vasculature, leading to a significant reduction in cardiac output and cerebral hypoperfusion. In some cases, it may be related to neuroreflex mechanisms.
Patients may present with various combinations of the above symptoms. Clinically, the so-called pulmonary infarction triad (dyspnea, chest pain, and hemoptysis) is sometimes observed but occurs in only about 20% of cases.
Signs
Tachypnea is common, and cyanosis may be observed. Lung auscultation may reveal wheezing and/or fine crackles, and occasionally vascular murmurs. Signs of atelectasis and pleural effusion may also be present if these conditions coexist.
In the circulatory system, findings are mainly related to pulmonary hypertension, right heart dysfunction, and acute reduction in left ventricular output. Sinus tachycardia is most common, and arrhythmias such as premature contractions, supraventricular tachycardia, atrial flutter, and atrial fibrillation may also occur. Some patients may have an accentuated or split second heart sound in the pulmonary valve area (P2 > A2) or a systolic ejection murmur. Jugular vein distension or abnormal pulsation, a systolic murmur in the tricuspid area, right ventricular gallop rhythm, hepatomegaly, positive hepatojugular reflux, and lower extremity edema are signs of right heart failure. A pericardial friction rub may be heard in rare cases. Severe cases may present with hypotension or even shock.
Fever, often low-grade, may occur. A small number of patients may have a fever exceeding 38°C, which can result from pulmonary infarction, pulmonary hemorrhage, atelectasis with secondary infection, or thrombophlebitis of the lower extremities.
Clinical Manifestations of DVT
DVT typically presents with swelling of the affected limb, increased circumference, pain or tenderness, and skin pigmentation. The affected limb may feel fatigued or have worsened swelling after walking. Asymmetric swelling of the lower limbs should raise suspicion. Measurement of the circumference of both lower limbs can help evaluate differences. The measurement points are 15 cm above the upper edge of the patella for the thigh and 10 cm below the lower edge of the patella for the calf. A difference of >1 cm between the two sides is considered clinically significant. More than half of lower limb DVT cases may have no subjective symptoms or obvious signs.
Diagnosis
The diagnostic process typically involves three steps: suspicion, confirmation, and identifying the cause.
Suspecting PTE (Suspicion)
When a patient presents with the aforementioned clinical manifestations, particularly in the presence of risk factors, such as unexplained dyspnea, chest pain, syncope, or shock, or with asymmetrical lower limb swelling, pain, etc., the following tests should be performed:
Plasma D-Dimer
The sensitivity of D-dimer for diagnosing acute PTE ranges from 92% to 100%, making it highly valuable for ruling out PTE in patients with low clinical probability. High-sensitivity D-dimer assays, such as enzyme-linked immunosorbent assay (ELISA), enzyme-linked immunofluorescence assay, high-sensitivity quantitative microparticle agglutination, and immunochemical luminescence analysis, are commonly used. A D-dimer level below 500 μg/L effectively rules out acute PTE.
The specificity of D-dimer decreases with age. Age-adjusted thresholds for D-dimer (for patients >50 years: age × 10 μg/L) can improve specificity. Conditions such as malignancy, inflammation, hemorrhage, trauma, surgery, or necrosis may elevate D-dimer levels, necessitating dynamic monitoring and clinical interpretation.
Arterial Blood Gas Analysis
Pulmonary vascular obstruction of more than 15% can cause hypoxemia. Acute PTE often presents with hypoxemia, hypocapnia, and an increased alveolar-arterial oxygen gradient (P(A−a)O2). However, some patients may have normal results.
Plasma Cardiac Troponins
Cardiac troponin I (cTnI) and troponin T (cTnT) may be elevated in acute PTE complicated by right ventricular dysfunction (RVD). Higher levels indicate more severe myocardial injury and a poorer prognosis.
Brain Natriuretic Peptide (BNP) and N-Terminal proBNP (NT-proBNP)
BNP and NT-proBNP are cardiogenic hormones synthesized and released by ventricular myocytes under increased pressure or ventricular dilation. In acute PTE, increased right ventricular afterload and wall tension raise BNP and NT-proBNP levels. Elevated levels reflect the severity of right ventricular dysfunction and hemodynamic disturbance. Elevated BNP or NT-proBNP levels in patients without underlying cardiac disease suggest the possibility of PTE and can also help assess prognosis.
Electrocardiogram (ECG)
Most cases show nonspecific ECG abnormalities. Common findings include sinus tachycardia, T-wave changes, and ST-segment abnormalities in leads V1-V4. Some cases exhibit the SIQIIITIII pattern (deep S wave in lead I, Q/q wave and inverted T wave in lead III). Other changes include complete or incomplete right bundle branch block, P pulmonale, right axis deviation, and clockwise rotation. Dynamic ECG changes have greater diagnostic value than static abnormalities for PTE.
Echocardiography
Echocardiography is valuable for diagnosing PTE, detecting right ventricular dysfunction, and ruling out other cardiovascular diseases. It can reveal signs of increased right ventricular afterload and, in some suspected PTE cases, thrombi in the right atrium, right ventricle, or pulmonary arteries.
Patients with suspected PTE can be stratified into different levels of clinical probability using various scoring systems. The most commonly used are the revised Geneva score and Wells score.
Confirming the Diagnosis (Confirmation)
Diagnostic imaging for PTE includes computed tomography pulmonary angiography (CTPA), ventilation/perfusion (V/Q) scanning, magnetic resonance pulmonary angiography (MRPA), and pulmonary angiography. Imaging for confirming DVT includes compression venous ultrasound, CT venography, radionuclide venography, and conventional venography.
CT Pulmonary Angiography (CTPA)
CTPA directly visualizes thrombi in the pulmonary arteries, showing their morphology, location, and degree of vascular obstruction. It is currently the first-choice diagnostic method for confirming PTE. Direct signs include filling defects within the pulmonary arteries, partially or completely surrounded by contrast-enhanced blood flow (railway track sign), or complete filling defects with non-visualization of distal vessels. Indirect signs include wedgy or banded increased lung density, discoid atelectasis, central pulmonary artery dilation, and reduced or absent distal vascular branches.
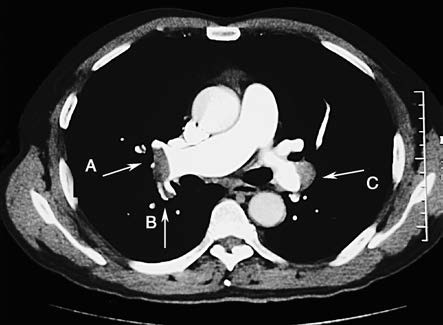
Figure 2 CTPA (right pulmonary artery view)
Thrombus in the distal right pulmonary artery (A) extending into the dorsal segmental artery of the right lower lobe (B); mural thrombus on the outer wall of the distal left pulmonary artery (C).
V/Q Scanning
The classic finding is perfusion defects in a lobar or segmental distribution that do not match ventilation imaging. V/Q scanning is particularly valuable for diagnosing distal PTE. However, many diseases can simultaneously affect pulmonary ventilation and perfusion, complicating the interpretation of V/Q scan results, which must be closely correlated with clinical findings. V/Q scanning is preferred for outpatients with low clinical probability, young patients (especially females), pregnant patients, those allergic to contrast agents, and patients with severe renal impairment.
Magnetic Resonance Pulmonary Angiography (MRPA)
MRPA can directly visualize emboli in the pulmonary arteries and areas of hypoperfusion caused by PTE. However, its diagnostic value is limited for PTE in the infrasegmental levels. MRPA has no radiation exposure, does not use iodine-based contrast agents, and allows imaging in any orientation. However, it requires advanced equipment and techniques, is time-consuming, and is less accessible. MRPA may be considered for patients with severe renal impairment, iodine contrast allergy, or pregnancy.
Pulmonary Angiography
Pulmonary angiography is the gold standard for diagnosing PTE, with high sensitivity and specificity. Direct signs include intravascular filling defects, with or without the railway track sign, and blood flow obstruction. Indirect signs include slow contrast flow, regional hypoperfusion, and delayed venous return. A diagnosis of PTE cannot be established in the absence of direct signs. Pulmonary angiography is an invasive procedure with a risk of fatal or severe complications of 0.1% and 1.5%, respectively, and should be performed only when clearly indicated.
For patients with suspected PTE, different diagnostic strategies are recommended based on the presence or absence of hemodynamic instability.
In hemodynamically unstable patients, if possible, CTPA can be performed to confirm or rule out PTE. If CTPA is unavailable or unsuitable, bedside echocardiography is recommended. If echocardiography shows evidence of increased right heart load and/or thrombi in the pulmonary arteries or right heart chambers, and other diseases are excluded, treatment for PTE should be initiated.
In hemodynamically stable patients, CTPA is the preferred diagnostic method. For patients with relative contraindications to CTPA (e.g., contrast allergy, renal impairment, pregnancy), alternative imaging modalities such as V/Q scanning or MRPA are recommended.
Identifying the Cause and Risk Factors (Etiological Diagnosis)
Detecting DVT
For any suspected PTE case, regardless of the presence of DVT symptoms, compression ultrasound of the lower extremities should be performed to confirm the presence of DVT and identify the source of emboli.
Identifying Triggers for DVT and PTE
Potential triggers such as immobilization, trauma, long-term use of oral contraceptives, or prothrombotic conditions like antiphospholipid syndrome should be investigated. Antiphospholipid antibody testing includes lupus anticoagulant, anticardiolipin antibodies, and anti-β2 glycoprotein 1 antibodies. A diagnosis of antiphospholipid syndrome requires both clinical criteria and laboratory criteria, including positive antiphospholipid antibody tests at least 12 weeks apart, excluding false positives caused by anticoagulants.
Hereditary thrombophilia, particularly in patients younger than 50 years, those with recurrent PTE, or those with a strong family history of venous thromboembolism (VTE), can be considered.
For patients with unexplained PTE or suspected hereditary thrombophilia (early onset, recurrence, unusual VTE locations, family history), genetic testing may be considered. For older patients, the possibility of underlying malignancy should be considered, with close follow-up.
Clinical Classification
Acute Pulmonary Thromboembolism
High-risk PTE
Hemodynamic instability indicates high-risk PTE. When clinical symptoms are accompanied by significant right heart failure and hemodynamic instability, there is a high risk of early mortality (within 30 days or during hospitalization).
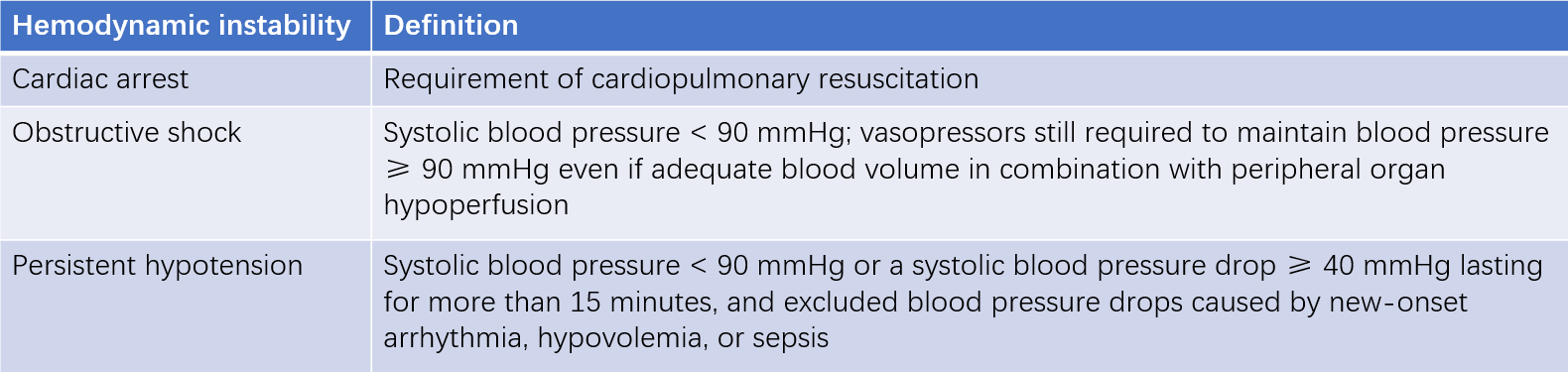
Table 2 Definition of Hemodynamic Instability
Intermediate-risk PTE
This is hemodynamically stable, but with imaging evidence of right ventricular dysfunction (RVD) and/or elevated cardiac biomarkers.
- Intermediate-high risk: Both RVD and elevated cardiac biomarkers are present.
- Intermediate-low risk: Either RVD or elevated cardiac biomarkers is present.
Diagnostic criteria for RVD:
- Imaging evidence includes echocardiography or CT findings indicating RVD. Echocardiographic findings consistent with RVD include:
- Right ventricular dilation (end-diastolic diameter of the right ventricle/left ventricle > 1.0).
- Reduced motion of the free wall of the right ventricle.
- Increased tricuspid regurgitation velocity.
- Reduced tricuspid annular plane systolic excursion (TAPSE < 17 mm).
- CTPA findings consistent with RVD include right ventricular dilation observed in the four-chamber view (end-diastolic diameter of the right ventricle/left ventricle > 1.0).
Cardiac biomarkers include BNP, NT-proBNP, and troponins.
Low-risk PTE
This is hemodynamically stable, with no evidence of RVD or elevated cardiac biomarkers.
Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
CTEPH is characterized by chronic and progressively worsening pulmonary hypertension, presenting with clinical features such as worsening dyspnea, fatigue, and reduced exercise tolerance. Advanced stages may lead to right heart failure. Imaging confirms pulmonary artery obstruction, often involving multiple, extensive areas of blockage. Chronic thrombotic signs include wall-adherent, eccentric, or calcified thrombi within the pulmonary arteries. DVT is often present.
At sea level, a resting mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg measured by right heart catheterization confirms pulmonary hypertension, provided other causes such as vasculitis or pulmonary artery sarcoma are excluded. Echocardiography may show right ventricular wall thickening, meeting the diagnostic criteria for chronic cor pulmonale.
Differential Diagnosis
Chest Pain
Acute Coronary Syndrome (ACS)
Some PTE patients may experience coronary ischemia and myocardial hypoxia, presenting as chest tightness or angina-like chest pain. ECG may show ischemic changes, which can lead to misdiagnosis as angina or myocardial infarction caused by coronary artery disease (CAD). Dynamic changes in ECG and cardiac enzyme levels, along with coronary angiography, can confirm CAD. Some patients may have coexisting PTE and CAD.
Aortic Dissection
This is frequently associated with hypertension and severe pain. Chest X-rays often show mediastinal widening, and cardiovascular ultrasound or CT angiography can reveal signs of aortic dissection.
Dyspnea
Pneumonia
When PTE presents with symptoms such as cough, hemoptysis, dyspnea, pleuritic chest pain, and imaging findings such as atelectasis or pulmonary opacities, especially with concurrent fever, pneumonia should be considered in the differential diagnosis.
Bronchial Asthma
PTE patients often experience chest tightness and shortness of breath, which necessitates differentiation from bronchial asthma.
Pleural Effusion
PTE patients may present with pleuritic chest pain and pleural effusion, which should be differentiated from pleural effusion caused by tuberculosis, pneumonia, malignancy, left heart failure, and other conditions.
Syncope
When PTE presents with syncope, it should be differentiated from syncope caused by vasovagal reflexes, cerebrovascular events, and arrhythmias.
Shock
Shock caused by PTE is classified as obstructive shock, characterized by low arterial pressure and elevated venous pressure. It should be differentiated from cardiogenic, hypovolemic, and distributive shock.
Hemoptysis
Hemoptysis occurs in various diseases. It is important to exclude hematemesis caused by gastrointestinal hemorrhage and differentiate PTE-related hemoptysis from conditions such as tuberculosis, bronchiectasis, malignancy, and left heart failure.
Other Pulmonary Artery Intracavitary Lesions
These include fat embolism syndrome, amniotic fluid embolism, air embolism, tumor embolism, and foreign body embolism. Additionally, differentiation should be made from primary malignant tumors of the pulmonary artery, pulmonary arteritis, and other conditions.
Treatment
General Supportive Care
Close monitoring of respiratory rate, heart rate, blood pressure, electrocardiogram (ECG), and blood gas changes is essential.
For patients with hypoxemia, oxygen therapy may be provided via nasal cannula, face mask, or high-flow nasal oxygen. In cases of respiratory failure, non-invasive mechanical ventilation or invasive mechanical ventilation through endotracheal intubation may be required.
Vasoactive drugs, such as dopamine, dobutamine, or norepinephrine, are often used to maintain effective hemodynamics and improve right heart function.
Sedatives and analgesics can be used appropriately. Attention should be given to maintaining smooth bowel movements to avoid straining.
Anticoagulation Therapy
Anticoagulation is considered the cornerstone of treatment for PTE. Once acute PTE is confirmed, it is typically initiated as early as possible.
Indications:
- Low-risk PTE patients are generally treated with anticoagulation therapy.
- High-risk PTE patients typically undergo thrombolytic therapy first, followed by anticoagulation.
- Intermediate-risk PTE patients are recommended to receive anticoagulation therapy regardless of whether thrombolysis is performed.
Contraindications and complications:
- Contraindications may include active bleeding or uncontrolled severe hypertension, though these are often not absolute contraindications in the context of acute PTE.
- Bleeding is the primary complication associated with anticoagulation therapy.
Unfractionated Heparin (UFH)
Intravenous administration is preferred, with an initial bolus of 2,000-5,000 U or 80 U/kg, followed by continuous infusion at 18 U/(kg·h). Dosage adjustments are made based on activated partial thromboplastin time (APTT) monitoring.
Platelet counts are monitored during treatment to detect heparin-induced thrombocytopenia (HIT).
Low-molecular-weight Heparin (LMWH)
Dosage varies by the specific LMWH preparation and is generally weight-based, and it can be administered subcutaneously 1-2 times daily.
Platelet counts are also monitored during treatment. Caution is advised in patients with renal impairment, as LMWH is cleared by the kidneys.
Fondaparinux
It is a selective factor Xa inhibitor that inhibits factor Xa by binding specifically to antithrombin.
Dosage is determined by body weight: 5 mg once daily (for body weight < 50 kg), 7.5 mg once daily (for body weight 50-100 kg), and 10 mg once daily (for body weight > 100 kg), administered subcutaneously. Dosage adjustments may be necessary for patients with impaired renal function.
Transition to oral anticoagulants is generally recommended after initiating parenteral anticoagulation, depending on the clinical condition.
Warfarin
This remains the most commonly used oral anticoagulant. The initial dose is typically 3.0-5.0 mg daily, with a lower starting dose of 2.5-3.0 mg daily for patients aged over 75 years or those at high risk of hemorrhage.
The international normalized ratio (INR) is monitored, with a target range of 2.0-3.0. Once stabilized, INR monitoring frequency is usually reduced to every 4-12 weeks.
Direct Oral Anticoagulants (DOACs)
These include direct factor Xa inhibitors (e.g., rivaroxaban, apixaban, edoxaban) and direct thrombin inhibitors (e.g., dabigatran).
In cases of hemorrhage, immediate discontinuation is generally recommended, and reversal agents such as prothrombin complex concentrate or fresh frozen plasma may be considered.
For confirmed acute PTE, a minimum duration of 3 months of anticoagulation therapy is typically recommended.
Patients with transient or reversible risk factors may discontinue anticoagulation in 3 months if acute PTE has resolved.
For patients without transient or reversible risk factors, or those with recurrent VTE (e.g., multiple episodes of PTE or DVT), antiphospholipid syndrome, or hereditary thrombophilia, extended anticoagulation therapy is often advised.
For patients with unprovoked PTE or persistent risk factors, prolonged anticoagulation therapy may be considered, with further evaluation of underlying risk factors.
For PTE patients with malignancy, long-term or even lifelong anticoagulation therapy is often recommended until the malignancy is fully resolved.
Thrombolytic Therapy
The thrombolysis window is generally considered to be within 14 days. Thrombolysis is typically performed cautiously and only after confirming the diagnosis of acute PTE.
Thrombolysis is primarily indicated for high-risk acute PTE, where it is often initiated promptly unless contraindications are present.
Routine systemic thrombolysis is not generally recommended for intermediate-risk PTE. Decisions are often based on an individualized assessment of benefits and risks.
Thrombolysis is not recommended for low-risk PTE. Rescue thrombolysis is usually considered for patients with hemodynamic deterioration during anticoagulation therapy.
Contraindications to thrombolysis are categorized as absolute or relative. For life-threatening high-risk PTE, absolute contraindications may be treated as relative.
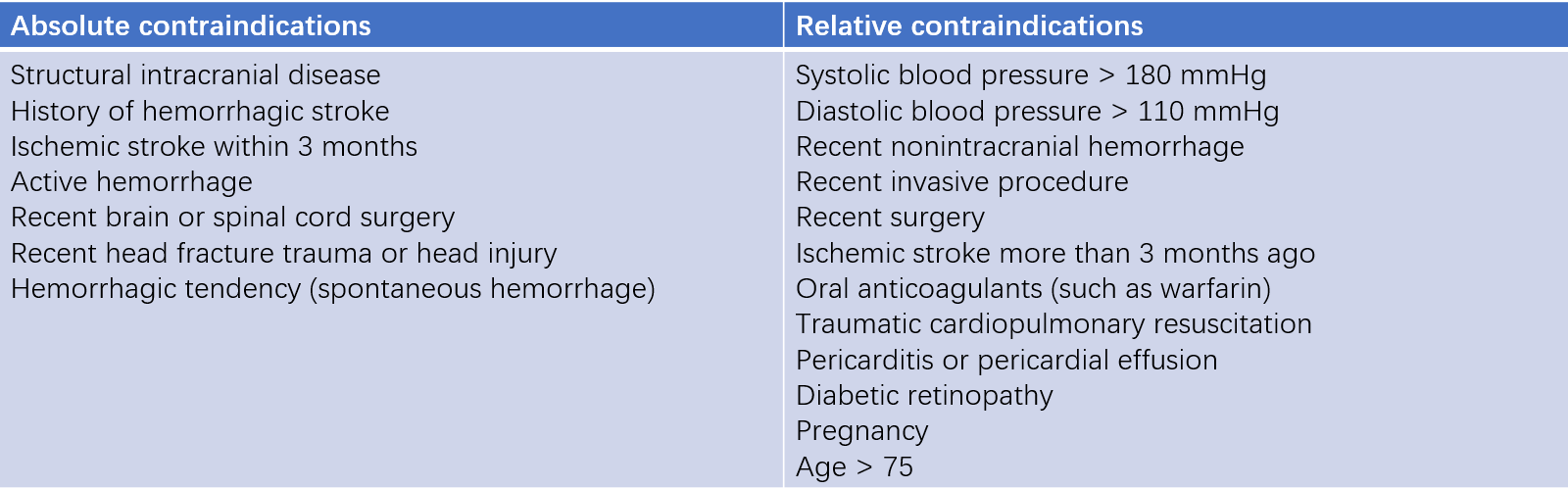
Table 3 Contraindications for thrombolysis
Common Thrombolytic Agents
Urokinase, streptokinase, and recombinant tissue plasminogen activator (rt-PA) are commonly used.
Low-dose thrombolysis (e.g., 50 mg rt-PA) is associated with similar efficacy but improved safety compared to the FDA-recommended dose (100 mg rt-PA).
APTT is typically measured 2 hours after thrombolysis. If APTT is less than twice the normal value, standard anticoagulation therapy (preferably UFH) is usually resumed.
Hemorrhage is the primary complication of thrombolysis. Risk assessments are often conducted prior to treatment, and blood products are prepared if necessary. Other adverse effects may include fever, allergic reactions (more frequent with streptokinase), hypotension, nausea, emesis, myalgia, and headache.
Interventional Therapy
Interventional therapy aims to remove thrombi, restore right heart function, and improve symptoms and survival rates.
Techniques include catheter-directed thrombus fragmentation and aspiration, often combined with localized low-dose thrombolysis.
Potential complications include distal embolization, pulmonary artery perforation, pulmonary hemorrhage, cardiac tamponade, conduction block or bradycardia, hemolysis, renal impairment, and complications related to vascular access.
Routine placement of inferior vena cava (IVC) filters is not generally recommended for most acute PTE patients. For patients with contraindications to anticoagulation, temporary IVC filters may be considered to prevent large lower extremity DVTs from embolizing to the lungs. Retrievable filters are typically recommended and are often removed within 2-4 weeks.
Surgical Therapy
Pulmonary embolectomy may be considered as a salvage option for patients with acute high-risk PTE who do not respond to medical or interventional therapies.
Treatment of Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
Treatment strategies include basic care, surgical therapy, pharmacological therapy, and interventional therapy.
Long-term anticoagulation, home oxygen therapy, cardiac function improvement, and rehabilitation are commonly implemented.
Pulmonary endarterectomy is often the preferred treatment for proximal pulmonary artery obstructions that are surgically accessible.
Balloon pulmonary angioplasty may be considered for distal lesions.
For patients who are inoperable, have residual pulmonary hypertension after surgery, or cannot undergo interventions, targeted therapy for pulmonary hypertension may be considered.
Prevention
Early identification of high-risk patients and timely preventive measures are considered effective in reducing the incidence of hospital-acquired VTE. Risk assessments typically take into account factors such as disease severity, age, and coexisting risk factors.
Basic Prevention
Health education, increased physical activity, and adequate hydration are commonly emphasized.
Prophylaxis
For patients at high risk of VTE with low hemorrhage risk, LMWH, UFH, fondaparinux, or DOACs are often used. Regular evaluations of effectiveness and hemorrhage risk are generally recommended during long-term prophylaxis.
Mechanical Prophylaxis
Mechanical methods such as intermittent pneumatic compression, graduated compression stockings, or foot venous pumps are often used for patients at high risk of VTE with active hemorrhage or high hemorrhage risk.