The pleural cavity is a potential space located between the lungs and the chest wall. Under normal circumstances, the visceral and parietal pleura are covered by a thin layer of fluid that serves as a lubricant during respiratory movements. Throughout each respiratory cycle, the shape and pressure of the pleural cavity undergo significant changes, allowing continuous filtration and absorption of pleural fluid, maintaining a dynamic balance. When fluid production in the pleural cavity exceeds absorption, or when absorption is impaired, pleural effusion occurs.
Mechanism of Pleural Fluid Circulation
The production and absorption of pleural fluid primarily occur on the surface of the parietal pleura. Under normal conditions, the volume of pleural fluid depends on the balance of hydrostatic and oncotic pressures between the parietal pleura, visceral pleura, and the pleural cavity. The blood supply of the parietal pleura originates from the systemic circulation, while the visceral pleura is supplied by both the pulmonary circulation and the bronchial arteries of the systemic circulation. Due to the higher pressure in the systemic circulation compared to the pulmonary circulation, fluid enters the interstitium from the systemic vessels of the parietal and visceral pleura. Some of this fluid is reabsorbed within the interstitium, while the remainder filters through the leaky mesothelial cell layer of the pleura into the pleural cavity. The fluid is then reabsorbed through the lymphatic stomata of the parietal pleura into the lymphatic system.
The formation of pleural effusion is regulated by the balance of various pressures. The hydrostatic pressure in the capillaries of the parietal pleura is similar to that of the systemic circulation, approximately 30 cmH2O, while that of the visceral pleura is about 24 cmH2O. The oncotic pressure in the capillaries of both the parietal and visceral pleura is 34 cmH2O. The intrapleural pressure is approximately -5 cmH2O, and the pleural fluid contains a small amount of protein, with an oncotic pressure of 5 cmH2O. The forces driving fluid filtration into the pleural cavity include hydrostatic pressure, intrapleural pressure, and the oncotic pressure of the pleural fluid, while the opposing force is the oncotic pressure of the capillaries. The net filtration pressure gradient for the parietal pleura is calculated as:
Hydrostatic pressure + Intrapleural pressure + Pleural fluid oncotic pressure − Capillary oncotic pressure = 30 + 5 + 5 - 34 = 6 cmH2O. This gradient facilitates fluid filtration from the parietal pleura into the pleural cavity. For the visceral pleura, the gradient is: 24 + 5 + 5 - 34 = 0 cmH2O. Thus, the visceral pleura plays a minimal role in pleural fluid circulation. Filtration of pleural fluid is greater in the upper parts of the pleural cavity, while absorption predominantly occurs in the diaphragmatic and mediastinal pleura of the lower pleural cavity.
Pathogenesis
Pleural effusion is a common clinical condition caused by diseases of the lungs, pleura, or extrapulmonary organs. The common causes and mechanisms include:
Increased Hydrostatic Pressure in Pleural Capillaries
Elevated hydrostatic pressure in the systemic and/or pulmonary circulation can increase fluid filtration into the pleural cavity (from the systemic circulation) or reduce fluid absorption (from the pulmonary circulation). Excessive filtration from the capillaries of the parietal pleura, surpassing the capacity for reabsorption, leads to pleural effusion. Common clinical conditions include congestive heart failure, constrictive pericarditis, increased blood volume, and obstruction of the superior vena cava or azygos vein, resulting in transudative pleural effusion.
Decreased Oncotic Pressure in Pleural Capillaries
Reduced plasma oncotic pressure due to hypoalbuminemia decreases the oncotic pressure in the capillaries of the parietal pleura, increasing fluid filtration. Simultaneously, reduced oncotic pressure in the visceral pleura capillaries decreases fluid reabsorption, leading to more pleural effusion. Common clinical conditions include hypoproteinemia, liver cirrhosis, nephrotic syndrome, acute glomerulonephritis, and myxedema, resulting in transudative pleural effusion.
Increased Pleural Permeability
Inflammation of the pleura or adjacent organs, or pleural tumors, can increase pleural capillary permeability due to direct pleural involvement or the release of enzymes, complements, and bioactive substances (e.g., histamine) by damaged cells. This results in the accumulation of protein-rich and cellular fluid in the pleural cavity, increasing oncotic pressure and further promoting fluid accumulation. Common clinical conditions include pleuritis (e.g., tuberculous pleuritis, parapneumonic effusion), connective tissue diseases (e.g., systemic lupus erythematosus, rheumatoid arthritis), pleural tumors (e.g., metastatic malignancy, mesothelioma), pulmonary infarction, and subdiaphragmatic inflammation (e.g., subdiaphragmatic abscess, liver abscess, acute pancreatitis), leading to exudative pleural effusion.
Obstruction of Parietal Pleural Lymphatic Drainage
Fluid and proteins in the pleural cavity return to the circulation via the lymphatic system. Malignant lymphatic obstruction, congenital lymphatic abnormalities, or trauma-induced lymphatic drainage impairment can result in protein-rich pleural effusion.
Trauma
Conditions such as rupture of the aorta, esophagus, or thoracic duct can lead to hemothorax, empyema, or chylothorax.
Iatrogenic Causes
Medications, radiation therapy, endoscopic procedures, bronchial artery embolization, ovarian hyperstimulation syndrome, fluid overload, coronary artery bypass surgery, bone marrow transplantation, central venous catheterization, and peritoneal dialysis can result in exudative or transudative pleural effusion.
Clinical Manifestations
Symptoms
Symptoms depend on the volume of pleural effusion. Small effusions (<0.3-0.5 L) are often asymptomatic. Large effusions commonly cause dyspnea, which is the most frequent symptom, often accompanied by chest pain and cough. As the volume of pleural effusion increases, chest pain may subside, but chest tightness and shortness of breath worsen.
Symptoms vary depending on the underlying disease. For example, tuberculous pleuritis is more common in young individuals and is often accompanied by fever, dry cough, and chest pain. Pleural effusion caused by heart failure may present with signs of cardiac dysfunction. Right-sided pleural effusion associated with liver abscess may manifest as reactive pleuritis or empyema, with fever and liver pain.
Signs
Physical signs depend on the volume of pleural effusion. Small effusions may present with no obvious signs or may produce pleural friction rubs. Moderate to large effusions may cause fullness of the affected hemithorax, reduced tactile fremitus, dullness on percussion, and diminished or absent breath sounds. Tracheal and mediastinal shift toward the contralateral side may occur. Pleural effusion caused by extrapulmonary diseases, such as rheumatoid arthritis or Sjögren's syndrome, is often accompanied by signs of the primary disease.
Laboratory and Other Examinations
Imaging Studies
Chest X-ray
X-ray findings vary depending on the volume of effusion, encapsulation, or adhesions. A lateral chest X-ray is particularly important for diagnosing small pleural effusions. Small, free-flowing pleural effusions may appear as blunting or obliteration of the costophrenic angle on a frontal X-ray. As the effusion increases, it forms a curved opacity with an upward and outward arc. In cases of large effusions, the affected hemithorax appears as a dense opacity, with the trachea and mediastinum shifted toward the contralateral side. In the supine position, the effusion spreads out, reducing the overall transparency of the lung field. In cases of hydropneumothorax, an air-fluid level is observed. Encapsulated effusions do not shift with changes in body position, have smooth and well-defined edges, and are often localized between lung lobes or between the lung and diaphragm. Subpulmonary effusions may present only as diaphragmatic elevation or changes in its contour.
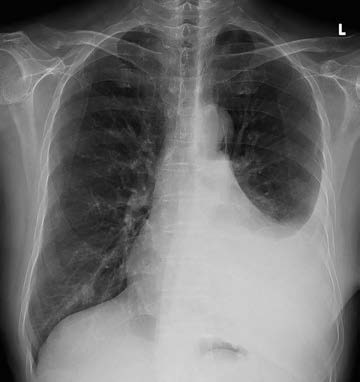
Figure 1 Chest X-ray showing left-sided pleural effusion.
Chest CT
Chest CT scans can detect small pleural effusions and help differentiate and localize encapsulated effusions. CT imaging also provides detailed visualization of abnormalities in the lungs, pleura, diaphragm, hilar structures, and mediastinum, aiding in the determination of the underlying cause and assessment of effusion volume.
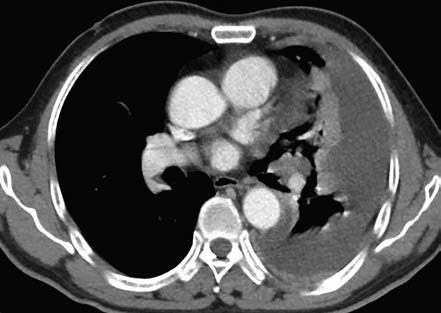
Figure 2 Chest CT scan showing left-sided pleural effusion.
Ultrasound
Ultrasound is highly sensitive in detecting pleural effusions and can estimate the depth and volume of effusion. It is useful for identifying encapsulation or septation and for guiding thoracentesis, reducing procedural risks.
Magnetic Resonance Imaging (MRI)
MRI provides high-resolution imaging of soft tissues and can assist in distinguishing between benign and malignant pleural effusions. It is particularly useful for patients allergic to contrast agents used in enhanced CT imaging.
PET-CT
PET-CT is a functional imaging technique widely used in oncology. It is based on differences in glucose metabolism between normal and abnormal tissues. Increased uptake of fluorine-18 fluorodeoxyglucose by tumor cells can assist in distinguishing between benign and malignant pleural effusions. PET-CT also aids in tumor staging and identifying the primary lesion.
Laboratory Tests
General Characteristics
Transudative effusions are typically pale yellow, clear, and non-coagulating when left standing, with a specific gravity of <1.016-1.018. Exudative effusions may appear slightly turbid, often forming clots, with a specific gravity of >1.018. Hemorrhagic pleural effusion appears as blood-tinged or resembles venous blood, commonly seen in malignancies, tuberculosis, or pulmonary embolism. Milky pleural effusions suggest chylothorax, often caused by tumors, parasitic infections, trauma (e.g., thoracic surgery or chest trauma), or tuberculosis leading to thoracic duct obstruction or rupture. Chocolate-colored effusions may indicate an amebic liver abscess rupturing into the pleural cavity. Black pleural effusions may suggest Aspergillus infection. Yellowish green effusions are often associated with rheumatoid arthritis.
Pleural effusions caused by anaerobic infections often have a foul smell, suggesting empyema. A urine-like odor suggests urinothorax, where creatinine levels in the pleural fluid exceed those in serum.
Cell Count and Differentiation
Inflammatory cells, as well as proliferative and degenerative mesothelial cells, may be observed in pleural effusions. Transudative effusions typically have fewer than 100x106 /L cells, while exudative effusions often exceed 500×106/L nucleated cells. In empyema, nucleated cell counts can exceed 10×109/L. However, there is no definitive cutoff between transudative and exudative effusions, requiring comprehensive analysis.
Neutrophil predominance is common in acute inflammation. Lymphocyte-predominant effusions are more frequently observed in tuberculosis, congestive heart failure, and malignancies. Eosinophilic effusions (≥10% eosinophils) may occur in parasitic infections, eosinophilic disorders, asbestosis, drug reactions, or malignancies.
Biochemical Analysis
Normal pleural fluid has a pH close to 7.6. A decreased pH is observed in empyema, esophageal rupture, and rheumatoid arthritis. Tuberculous and malignant effusions may also have a reduced pH, with values below 7.0 typically seen only in empyema or esophageal rupture.
Glucose levels in normal pleural fluid are similar to those in peripheral blood. Transudative and most exudative effusions have normal glucose levels. Low glucose levels may be seen in tuberculosis, malignancy, lupus pleuritis, and complicated parapneumonic effusions. Markedly reduced glucose (<3.3 mmol/L) is most commonly associated with empyema, complicated parapneumonic effusions, or rheumatoid arthritis.
Exudative effusions have high protein content (>30 g/L) with a pleural fluid/serum protein ratio >0.5. Transudative effusions have low protein content (<30 g/L), predominantly albumin, and a negative Rivalta test.
Elevated lactate dehydrogenase (LDH) levels are characteristic of exudative effusions, with a pleural fluid/serum LDH ratio >0.6. LDH levels >500 IU/L suggest malignancy or pleural infection. Adenosine deaminase (ADA), an enzyme involved in T-lymphocyte differentiation, is often elevated (>45 IU/L) in tuberculous pleuritis. In AIDS patients with tuberculous pleuritis, ADA levels are often <40 IU/L. Elevated ADA may also occur in empyema, rheumatoid arthritis, lupus pleuritis, and malignant effusions. Isoenzymes (ADA1 and ADA2) can help differentiate tuberculous from non-tuberculous causes. Elevated amylase levels may indicate acute pancreatitis, esophageal rupture, or malignancy.
Chylous effusions contain high levels of triglycerides (>1.24 mmol/L), with their composition influenced by dietary intake. Sudan III staining is positive, while cholesterol levels remain normal. Pseudochylous effusions have high cholesterol levels (>5.18 mmol/L) due to cholesterol accumulation but lack chylomicrons, with normal triglyceride levels and negative Sudan III staining. These are often associated with chronic tuberculous pleuritis or rheumatoid pleuritis.
Tumor Markers
Carcinoembryonic antigen (CEA) is a tumor-associated marker. Pleural fluid CEA >10 μg/L or a pleural fluid/serum CEA ratio >1 strongly suggests malignant pleural effusion, with high specificity but low sensitivity. CEA is particularly valuable for diagnosing adenocarcinomas, especially CEA-secreting lung adenocarcinomas, gastrointestinal tumors, and breast cancer. Other tumor markers, such as CA125, CYFRA 21-1, and neuron-specific enolase, may provide additional diagnostic information.
Immunological Tests
Elevated levels of interferon-gamma (IFN-γ) and IL-27 in pleural fluid are highly sensitive and specific for tuberculous pleuritis. IFN-γ release assays (IGRAs) measure IFN-γ levels produced by T cells in response to Mycobacterium tuberculosis-specific antigens, but their sensitivity and specificity in pleural fluid are limited.
In systemic lupus erythematosus and rheumatoid arthritis, pleural fluid complement levels (C3, C4) are reduced, while immune complex levels are elevated. ANA titers in lupus pleuritis often exceed 1:160, and rheumatoid factor levels in rheumatoid pleuritis may exceed 1:320.
Pathogen Identification
Acid-fast staining of pleural fluid has a low positivity rate (<10%). The yield of Mycobacterium tuberculosis culture depends on the medium used, with liquid media offering higher sensitivity and shorter turnaround times than solid media. Nucleic acid amplification tests for Mycobacterium tuberculosis in pleural fluid have a specificity >90% but low sensitivity. Xpert MTB/RIF has high specificity (99%) but limited sensitivity (37%-51%) for diagnosing tuberculous pleuritis.
Bacterial smears, cultures, and nucleic acid amplification tests can aid in identifying pathogens in pleural infections. Culturing pleural fluid in blood culture bottles improves detection rates. Metagenomic next-generation sequencing (mNGS) can rapidly identify various pathogens, including Nocardia, Pneumocystis, Mycobacterium tuberculosis, Enterobacteriaceae, Streptococcus, Fusobacterium, and Porphyromonas. While mNGS is more sensitive than conventional methods, its lower specificity necessitates clinical correlation.
Cytological Examination
Malignant cells are detected in 40%-87% of patients with malignant pleural effusion, depending on the type and location of the primary tumor and the quality of sample collection. Repeated examinations can improve detection rates.
Pleural Biopsy
Percutaneous pleural biopsy is valuable for diagnosing the etiology of pleural effusions and can identify tumors, tuberculosis, and other granulomatous pleural diseases. In suspected tuberculous pleuritis, biopsy specimens can be used for pathological examination, DNA testing for Mycobacterium tuberculosis, acid-fast staining, or culture. Pleural biopsy is simple, minimally invasive, and can be performed under CT or ultrasound guidance to improve success rates.
Thoracoscopy or Open Biopsy
If the above tests fail to establish a diagnosis, thoracoscopy or open biopsy may be performed. Thoracoscopy has a sensitivity of 92%-97% and specificity of 99%-100% for diagnosing malignant pleural effusions. It allows comprehensive examination of the pleural cavity, assessment of lesion morphology and extent, and targeted biopsies under direct visualization. In rare cases where the cause of pleural effusion remains unclear, exploratory thoracotomy may be considered if no contraindications exist.
Bronchoscopy
Bronchoscopy is useful in patients with hemoptysis or suspected airway obstruction, especially when lung cancer is suspected. However, its diagnostic yield is low in the absence of these abnormalities.
Diagnosis and Differential Diagnosis
The diagnosis and differential diagnosis of pleural effusion can be divided into three steps:
Determining the Presence of Pleural Effusion
Diagnosing moderate to large pleural effusions is not difficult, as symptoms and physical signs are often evident. Small pleural effusions may only present as blunting of the costophrenic angle, which can sometimes be confused with pleural adhesions. A lateral decubitus chest X-ray of the affected side may show fluid spreading along the lateral thoracic wall. Ultrasound and CT imaging can confirm the presence of pleural effusion.
Differentiating Transudative and Exudative Effusions
Diagnostic thoracentesis can help determine the nature of the effusion. Transudative effusions are clear, transparent, colorless, or pale yellow and do not coagulate. Exudative effusions often appear darker, ranging from transparent or turbid straw yellow to brownish yellow or bloody, and may coagulate spontaneously. Differentiation can be made based on specific gravity (threshold of 1.018), protein content (threshold of 30 g/L), and cell count (threshold of 500×106/L). Effusions below these thresholds are classified as transudative, while those exceeding them are classified as exudative, though the sensitivity and specificity of these criteria are relatively low.
The Light's criteria are commonly used to differentiate transudative from exudative effusions, primarily by measuring protein and lactate dehydrogenase (LDH) levels in pleural fluid. According to Light's criteria, the effusion is classified as exudative if any one of the three criteria is met; if none are met, the effusion is classified as transudative. However, Light's criteria have limited specificity for exudative effusions, with approximately 25% of transudative effusions misclassified as exudative.

Table 1 Differentiation between exudative and transudative effusions (Light's criteria).
Identifying the Cause of Pleural Effusion
Transudative Effusions
The main causes of transudative effusions include:
- Congestive heart failure: This is often bilateral, with more fluid on the right side. Aggressive diuresis may result in a pseudo-exudative effusion. NT-proBNP levels >1,500 pg/mL in pleural fluid have high diagnostic value for heart failure-related effusions.
- Nephrotic syndrome: This typically involves bilateral effusions, which may resolve as protein loss is corrected.
- Cirrhosis: Often accompanied by ascites, with pleural effusion usually on the right side.
- Other causes: These include acute glomerulonephritis, constrictive pericarditis, peritoneal dialysis, myxedema, drug allergies, and radiation reactions.
Exudative Effusions
Exudative effusions have a wide range of causes, with common ones including parapneumonic effusion, tuberculosis, and malignancy.
Parapneumonic effusion refers to pleural effusion caused by infections such as pneumonia, lung abscess, or bronchiectasis. Based on the mechanism, it can be classified into simple parapneumonic effusion and complicated parapneumonic effusion. Simple parapneumonic effusion results from reactive pleural exudation and resolves as the pneumonia improves. When bacteria invade the pleural cavity, it leads to complicated parapneumonic effusion. Symptoms often include fever, cough, sputum production, and chest pain, with elevated white blood cell counts, neutrophilia, and a left shift. Imaging (X-ray or CT) initially shows pulmonary infiltrates, lung abscesses, or bronchiectasis, followed by pleural effusion, which is usually small in volume. Complicated parapneumonic effusions are often yellow and turbid, with significantly elevated nucleated cell counts (predominantly neutrophils), reduced glucose and pH levels, and elevated LDH.
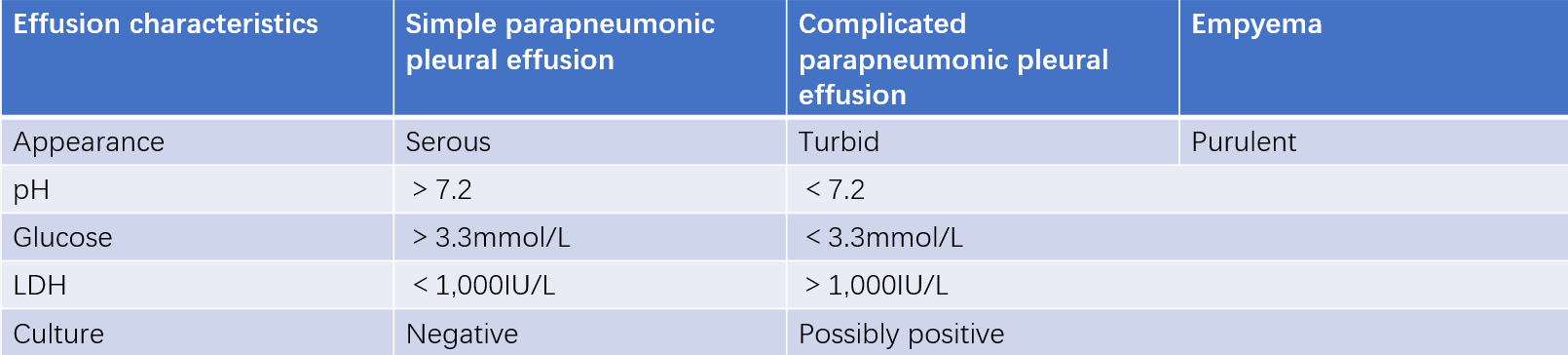
Table 2 Characteristics of parapneumonic effusion and empyema.
Empyema refers to pus accumulation in the pleural cavity due to infection. It is often associated with inadequately controlled pulmonary infections or direct bacterial invasion into the pleural space. Acute empyema presents with high fever and chest pain, while chronic empyema may involve pleural thickening, chest wall retraction, chronic wasting, and clubbing of fingers or toes. The pleural fluid is purulent and viscous, with potentially positive bacterial cultures.
Pleural infections include complicated parapneumonic effusions and empyema. Community-acquired pleural infections are mostly caused by Gram-positive aerobic bacteria, such as Streptococcus and Staphylococcus aureus. Gram-negative bacteria are less common. Hospital-acquired pleural infections are often caused by multidrug-resistant Gram-positive bacteria (e.g., MRSA) or Gram-negative bacteria (e.g., Enterobacteriaceae, Pseudomonas). Anaerobic bacteria are frequently involved, while fungal, Actinomyces, and Nocardia infections are rare.
Tuberculous pleuritis can occur at any age and is often associated with pulmonary or extrapulmonary tuberculosis. Symptoms include chest pain and shortness of breath, with systemic symptoms such as low-grade fever, night sweats, and weight loss. Tuberculin skin tests are usually positive or strongly positive. In elderly patients, fever may be absent, and tuberculin tests may be negative. The pleural fluid is often yellow, occasionally bloody, with lymphocyte predominance and mesothelial cells <5%. ADA, IFN-γ, and IL-27 levels are elevated. Acid-fast staining of pleural fluid has a low positivity rate (<10%), while Mycobacterium tuberculosis cultures yield positive results in 18%-40% of cases. Nucleic acid amplification tests and Xpert MTB/RIF can detect MTB DNA and rifampin resistance genes, with high specificity but low sensitivity.
Pleural biopsy, especially under CT or ultrasound guidance, is valuable for diagnosing tuberculous pleuritis. Pathology typically reveals granulomas, and biopsy specimens can also be tested for MTB DNA and acid-fast staining, with sensitivities of 60%-80%. Culture of biopsy tissue further improves sensitivity. Thoracoscopic pleural biopsy (pathology, DNA testing, and culture) has even higher sensitivity.
Malignant pleural effusion refers to effusion caused by primary pleural malignancies or metastatic cancers, including lung cancer, breast cancer, hematologic malignancies, gastrointestinal cancers, gynecologic cancers, and malignant mesothelioma. Malignant pleural effusion is a common complication of advanced cancer, typically progressing rapidly and persistently, with poor treatment outcomes and prognosis. It is more common in middle-aged and elderly individuals, presenting with symptoms such as dyspnea, dull chest pain, hemoptysis, and weight loss. The effusion is often bloody, large in volume, and rapidly increasing, with elevated tumor markers such as CEA and LDH (>500 IU/L). Imaging studies provide diagnostic clues, but definitive diagnosis requires pathological confirmation of malignant cells in pleural fluid or pleural biopsy specimens. Cytological examination of pleural fluid, pleural biopsy, bronchoscopy, and thoracoscopy can aid in diagnosis and differentiation. Further investigations are needed to evaluate tumors in other organs if suspected.
Treatment
Pleural effusion is often part of a systemic or thoracic disease, and treating the underlying cause is crucial. Transudative effusions usually resolve after addressing the underlying condition, while the management of exudative effusions varies depending on the cause.
Parapneumonic Effusion
Simple parapneumonic effusions are usually small and resolve with antibiotic therapy. The treatment of pleural infections (complicated parapneumonic effusion and empyema) focuses on infection control, drainage of pleural fluid, and nutritional support. Antibiotic selection should be based on whether the infection is community- or hospital-acquired, local microbial prevalence and resistance patterns, and patient-specific factors. The treatment duration is typically 2-6 weeks.
The decision to drain pleural fluid depends on pleural fluid biochemistry (e.g., pH, LDH, glucose) and imaging features (e.g., ultrasound, CT). Drainage methods include thoracentesis and chest tube placement. For recurrent or chronic infections, especially in cases of lung collapse or inoperable patients, chest tube placement should be considered. Chronic empyema may require surgical interventions such as pleural decortication or thoracoplasty. Supportive care, including a high-calorie, high-protein, and vitamin-rich diet, correction of electrolyte imbalances, and maintenance of acid-base balance, is also essential.
Tuberculous Pleuritis
The goals of treatment include controlling tuberculosis, minimizing residual pleural thickening after effusion absorption, preventing lung function impairment, and reducing pleural thickening-related complications.
General management includes rest, nutritional support, and symptomatic treatment.
Due to the high protein content of tuberculous pleural effusion, pleural adhesions are common. Prompt and complete drainage of pleural fluid via thoracentesis or chest tube placement is recommended to relieve cardiopulmonary compression, reduce fibrin deposition, and alleviate symptoms of tuberculosis toxicity. The initial aspiration volume should not exceed 800 mL, and subsequent aspirations should not exceed 1,000 mL each time to avoid complications such as re-expansion pulmonary edema or shock.
The principles of therapy are similar to those for active pulmonary tuberculosis, emphasizing early, consistent, full-course, adequate, and combination therapy.
The efficacy of corticosteroids is uncertain. In cases with severe systemic symptoms or large effusions, corticosteroids may be added to antituberculous therapy. Prednisone 20-30 mg/day is commonly used, with gradual tapering as symptoms improve, typically over 4-6 weeks.
For tuberculous empyema or bronchopleural fistula, most patients require surgical treatment. Preoperative chest tube drainage may help reduce the empyema cavity and prepare for pleural decortication or pleuropneumonectomy.
Malignant Pleural Effusion
Treatment includes addressing the underlying malignancy and managing the pleural effusion. Lung and breast cancers are the most common causes. Treating the primary tumor (e.g., chemotherapy, targeted therapy, anti-angiogenic agents, immunotherapy, or radiotherapy) may provide some benefit.
If the patient does not experience dyspnea, drainage of the effusion is unnecessary, and close monitoring while treating the primary tumor is sufficient. For patients with dyspnea, thoracentesis can determine whether symptoms are related to the effusion and assess lung re-expansion potential. In cases where the lung can re-expand, chest tube placement or pleurodesis may be first-line options. For non-expandable lungs, chest tube placement is preferred.
Intrathoracic administration of therapeutic agents, anti-angiogenic drugs, or biologics may help control effusion. Commonly used agents include cisplatin, lobaplatin, recombinant human endostatin, bevacizumab, interleukin-2, tumor necrosis factor, interferons, and tumor-infiltrating lymphocytes.