The pleural cavity is a closed potential space that does not normally contain gas. Under normal conditions, the pleural cavity is under negative pressure. Pneumothorax refers to the presence of air in the pleural cavity due to any cause. It can be classified into three types: spontaneous, traumatic, and iatrogenic. Spontaneous pneumothorax can be further divided into primary and secondary types. Primary spontaneous pneumothorax occurs in healthy individuals without underlying lung disease, while secondary spontaneous pneumothorax typically occurs in patients with pre-existing lung conditions. Traumatic pneumothorax results from direct or indirect chest wall injury caused by trauma, while iatrogenic pneumothorax is caused by diagnostic or therapeutic procedures. Pneumothorax is a common medical emergency, with a higher incidence in males than females. The incidence rate of primary spontaneous pneumothorax is 18-28 per 100,000 population in males and 1.2-6 per 100,000 population in females. Following the onset of pneumothorax, the negative pressure in the pleural cavity may become positive, leading to obstruction of venous return to the heart and varying degrees of cardiac and pulmonary dysfunction. This section primarily discusses spontaneous pneumothorax.
Etiology and Pathogenesis
Under normal circumstances, there is no gas in the pleural cavity because the total partial pressure of gases in pleural capillary blood is 706 mmHg, which is 54 mmHg lower than atmospheric pressure. The lower total gas pressure in pleural capillaries compared to atmospheric pressure prevents the presence of gas in the pleural cavity. Additionally, the outward expansion force of the chest wall and the inward elastic recoil of the lungs create negative pressure throughout the respiratory cycle. Gas can enter the pleural cavity under three conditions:
- A breach between the alveoli and the pleural cavity.
- A communication between the chest wall and the external environment due to trauma.
- The presence of gas-producing microorganisms in the pleural cavity.
The first two causes are most commonly observed clinically. Pneumothorax disrupts the negative pressure in the pleural cavity, and in some cases, positive pressure compresses the lung, impairing its ability to expand. This leads to reduced lung volume, decreased vital capacity, and lower maximal ventilatory capacity, resulting in restrictive ventilatory dysfunction. The reduced lung volume initially does not decrease pulmonary blood flow, causing a ventilation-perfusion mismatch and hypoxemia. In cases of large pneumothorax, the loss of negative pressure in the pleural cavity and the compression of blood vessels and the heart by positive intrapleural pressure reduce cardiac filling and output, leading to tachycardia, hypotension, or even shock. Tension pneumothorax can cause mediastinal shift, circulatory collapse, or asphyxiation.
Primary Spontaneous Pneumothorax (PSP)
This type occurs in healthy individuals without significant underlying lung disease. The rupture of subpleural blebs or bullae is thought to be the main mechanism. Studies suggest that smoking, body habitus, and family history are risk factors. PSP is more common in tall, thin, young males. Routine chest X-rays may not reveal significant lung abnormalities, but subpleural blebs, often located at the lung apex, may be present. The causes of these blebs are unclear but may be related to smoking, height, small airway inflammation, nonspecific inflammatory scarring, or congenital elastic fiber dysplasia.
Secondary Spontaneous Pneumothorax (SSP)
This is the most common type of pneumothorax and occurs in patients with underlying lung diseases. Conditions such as partial obstruction or distortion of small airways, forming a valve-like mechanism, can lead to overinflation of alveoli, destruction of alveolar walls, and the formation of bullae. A sudden increase in intrapulmonary pressure may cause the rupture of subpleural bullae, resulting in pneumothorax. Common underlying diseases include tuberculosis, chronic obstructive pulmonary disease (COPD), lung cancer, lung abscess, pulmonary fibrosis, eosinophilic granulomatosis, sarcoidosis, pneumoconiosis, and lymphangioleiomyomatosis. Due to the presence of underlying lung disease, SSP is associated with more severe clinical symptoms and poorer tolerance to pneumothorax compared to PSP, necessitating more active intervention in most cases.
Special Types of Pneumothorax
Catamenial Pneumothorax
This refers to recurrent pneumothorax associated with the menstrual cycle, accounting for 0.9% of spontaneous pneumothorax cases in females. It occurs within 24-72 hours before or after the onset of menstruation. The exact pathogenesis is unclear, but it is thought to result from the rupture of ectopic endometrial nodules in the lungs, pleura, or diaphragm.
Pregnancy-associated Pneumothorax
This type occurs during pregnancy, with episodes associated with each pregnancy. Based on the timing of onset, it can be classified into early (3-4 months of pregnancy) and late (after 8 months of pregnancy) types. The mechanism is unclear but may involve hormonal changes and alterations in thoracic compliance.
Clinical Classification
Spontaneous pneumothorax is typically classified into three types based on the condition of the pleural rupture and its effect on intrapleural pressure:
Closed (Simple) Pneumothorax
The pleural rupture is small and seals as the lung collapses, preventing further air entry into the pleural cavity. In this case, intrapleural pressure is close to or slightly above atmospheric pressure, and the pressure does not rise after air aspiration.
Open (Communicating) Pneumothorax
The rupture is large or remains open due to pleural adhesions or traction, allowing free movement of air in and out of the pleural cavity during inspiration and expiration. Intrapleural pressure fluctuates around 0 cmH2O. After aspiration, the pressure may become negative but often returns to pre-aspiration levels within minutes.
Tension (High-pressure) Pneumothorax
The rupture creates a one-way valve mechanism, allowing air to enter the pleural cavity during inspiration but preventing its exit during expiration. This leads to progressive air accumulation, increasing intrapleural pressure. The rising pressure compresses the lung, displaces the mediastinum to the contralateral side, and impairs venous return to the heart. Intrapleural pressure often exceeds 10 cmH2O and can reach 20 cmH2O or higher. Although air aspiration temporarily reduces pressure, it rapidly rebounds, causing significant respiratory and circulatory dysfunction that requires emergency management.
Clinical Manifestations
The severity of symptoms depends on the presence of underlying lung disease, the functional status of the lungs, the speed of pneumothorax onset, the volume of air in the pleural cavity, and the intrapleural pressure.
Symptoms
The severity of dyspnea is closely related to baseline lung function, the degree of lung collapse, and the speed of onset. Young individuals with normal lung function may have no significant dyspnea, while COPD patients with poor lung function may experience pronounced dyspnea even with mild lung compression. Chronic pneumothorax may present with mild shortness of breath due to compensatory function of the healthy lung. Dyspnea is more pronounced in cases with mediastinal emphysema, sometimes leading to cyanosis.
Chest pain often presents as sudden, sharp, stabbing, or knife-like pain on one side of the anterior chest or axilla. The pain is usually short-lived, worsens during inspiration, and may radiate to the shoulder, back, or upper abdomen. The severity of chest pain is not correlated with the degree of lung compression.
Irritative dry cough is caused by pleural irritation from the air and is usually mild, with little or no sputum, though there may be traces of blood.
Symptoms of SSP are generally more severe than those of PSP. In tension pneumothorax, the sudden increase in intrapleural pressure compresses the lungs and displaces the mediastinum, causing severe respiratory and circulatory distress. Patients may exhibit extreme anxiety, chest tightness, restlessness, cyanosis, perspiration, tachycardia, syncope, arrhythmias, altered consciousness, or respiratory failure.
Signs
Physical signs depend on the volume of air in the pleural cavity. Small pneumothoraces may have minimal signs, especially in patients with emphysema, but diminished breath sounds on auscultation are significant. In large pneumothoraces, the trachea may shift to the contralateral side. The affected chest may appear distended, with reduced chest wall movement and tactile fremitus. Percussion reveals hyperresonance or tympany, while cardiac or hepatic dullness may be reduced or absent. Breath sounds on the affected side are diminished or absent. In left-sided pneumothorax or mediastinal emphysema, a bubbling sound synchronous with the heartbeat (Hamman's sign) may sometimes be heard near the left cardiac border. In hydropneumothorax, splashing sounds may be present in the chest. In hemopneumothorax, significant blood loss can cause hypotension or hemorrhagic shock.
Imaging and Other Examinations
Chest X-ray Examination
An upright posteroanterior chest X-ray is an essential method for diagnosing pneumothorax. It can reveal the degree of lung compression, the presence of intrapulmonary lesions, pleural adhesions, pleural effusion, and mediastinal shift. The typical radiographic finding of pneumothorax is a thin, outwardly convex arc-shaped opacity, which represents the boundary between lung tissue and air in the pleural cavity, referred to as the pneumothorax line. Inside the line lies the compressed lung tissue, while outside the line is an area of increased radiolucency without lung markings. In cases of large pneumothorax, the lung may appear compressed near the hilum, forming a rounded opacity, often accompanied by mediastinal shift toward the contralateral side.
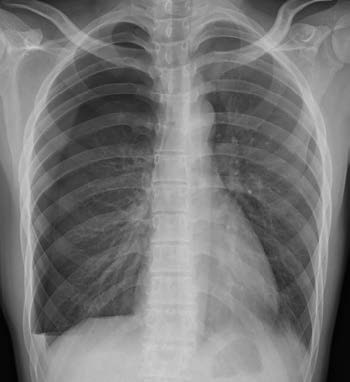
Figure 1 Frontal chest X-ray showing right-sided pneumothorax
In cases of pulmonary tuberculosis or chronic pulmonary inflammation, multiple pleural adhesions may be present, resulting in localized or encapsulated pneumothorax. Sometimes, pneumothorax may involve communication between different areas. When pneumothorax extends to the lower chest cavity, the costophrenic angle may become sharper. If pleural effusion is present, an air-fluid level can be observed. Localized pneumothorax may be missed on posteroanterior chest X-rays, but lateral chest X-rays can aid in diagnosis.
Clinically, the degree of lung compression after pneumothorax can be estimated using chest X-rays. For example, if the distance between the pneumothorax line and the apex of the thoracic cavity is ≥3 cm, it is considered a large pneumothorax; if <3 cm, it is classified as a small pneumothorax. At the level of the hilum, when the distance from the lateral chest wall to the lung edge is 1 cm, it corresponds to approximately 25% of the unilateral thoracic cavity volume; at 2 cm, it corresponds to about 50%. Therefore, a distance of ≥2 cm indicates a large pneumothorax, while <2 cm indicates a small pneumothorax.
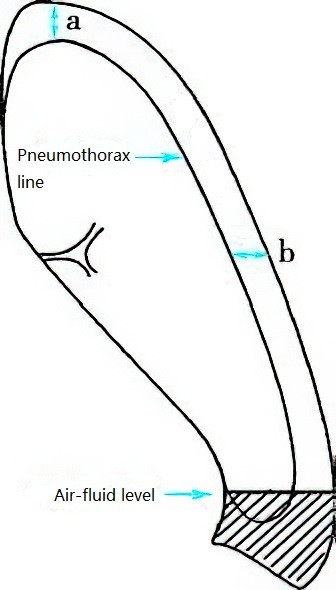
Figure 2 Method for measuring pneumothorax volume
Chest CT
Chest CT is more sensitive and accurate than chest X-rays in detecting small or localized pneumothorax and distinguishing between pulmonary bullae and pneumothorax. It also provides a more precise assessment of the volume of air in the pleural cavity. CT findings include the presence of extremely hypodense gas in the pleural cavity, accompanied by varying degrees of lung compression.
Arterial Blood Gas Analysis and Pulmonary Function Testing
Most patients with pneumothorax exhibit abnormal arterial blood gas analysis results, typically showing reduced PaO2, while PaCO2 is usually normal or decreased. Pulmonary function testing is of limited value in detecting pneumothorax or estimating its volume and is therefore not routinely recommended in clinical practice.
Diagnosis
Pneumothorax can generally be diagnosed based on clinical symptoms, physical findings, and imaging studies. The presence of a pneumothorax line on X-ray or CT imaging is a definitive diagnostic feature. In cases of sudden-onset dyspnea where the patient is critically ill and cannot be transported for imaging studies, diagnostic thoracentesis can be performed at the site of the most pronounced physical findings on the affected side. The aspiration of air confirms the diagnosis of pneumothorax.
Spontaneous pneumothorax, especially in older patients or those with pre-existing chronic heart or lung diseases, may mimic other acute cardiopulmonary conditions, requiring careful differentiation.
Differential Diagnosis
Asthma and Chronic Obstructive Pulmonary Disease (COPD)
Both conditions can present with varying degrees of dyspnea during acute exacerbations, and their physical findings may resemble those of spontaneous pneumothorax. Asthma patients often have a history of recurrent episodic wheezing, while COPD patients typically experience progressively worsening dyspnea over time. If a patient with asthma or COPD suddenly develops severe dyspnea, perspiration, restlessness, and does not respond to bronchodilators or antibiotics, pneumothorax should be suspected. Chest X-ray or CT imaging can assist in differentiation.
Acute Myocardial Infarction (AMI)
AMI can present with sudden chest pain, chest tightness, dyspnea, or even shock. A history of hypertension, atherosclerosis, or coronary artery disease is often present. Physical findings, electrocardiography (ECG), chest X-ray or CT imaging, and serum enzyme tests can help establish the diagnosis.
Acute Pulmonary Embolism (PE)
Massive PE can present with sudden onset of dyspnea, chest pain, restlessness, panic, or a sense of impending death, which clinically resembles spontaneous pneumothorax. However, PE may be accompanied by hemoptysis, low-grade fever, and syncope. A history of deep vein thrombosis, fractures, recent surgery, stroke, atrial fibrillation, or prolonged bed rest in older patients is often present. CT pulmonary angiography can aid in differentiation.
Pulmonary Bullae
Peripheral pulmonary bullae, especially giant bullae, can be mistaken for pneumothorax. Pulmonary bullae typically have a slow onset, and dyspnea is usually not severe, whereas pneumothorax often occurs suddenly. On imaging, pulmonary bullae appear as round or oval air-filled spaces with fine linear striations, representing remnants of pulmonary lobules or blood vessels. Bullae expand outward, compressing the lung toward the apex, costophrenic angle, or cardiophrenic angle. In contrast, pneumothorax appears as a radiolucent band along the chest wall without lung markings. Fluoroscopy from different angles can reveal the round radiolucent area of a bulla, without the fine pneumothorax line at its edge. The pressure inside a bulla is similar to atmospheric pressure, and its size does not change significantly after aspiration. Misguided aspiration of a bulla can lead to pneumothorax.
Other Conditions
Differential diagnoses include perforated peptic ulcer, pleuritis, costochondritis, lung cancer, and diaphragmatic hernia. These conditions may occasionally present with acute chest pain, upper abdominal pain, or dyspnea, mimicking spontaneous pneumothorax. Careful assessment is necessary to distinguish between these conditions.
Severity Assessment
For clinical observation and management, spontaneous pneumothorax can be categorized into stable and unstable types based on clinical presentation. A patient is considered stable if all of the following criteria are met; otherwise, the condition is classified as unstable:
- Respiratory rate <24 breaths/min.
- Heart rate of 60-120 beats/min.
- Normal blood pressure.
- Oxygen saturation (SaO2) >90% on room air.
- Ability to speak in full sentences between breaths.
Treatment
Treatment Goals
The primary goals of treatment include promoting lung re-expansion on the affected side, addressing the underlying cause, and reducing the risk of recurrence. Common treatment methods include conservative management, thoracic decompression and air evacuation (thoracentesis and closed thoracic drainage), pleurodesis, and surgical intervention. The specific treatment strategy for each patient should be appropriately selected based on the type and cause of pneumothorax, frequency of episodes, degree of lung compression, overall clinical condition, and presence of complications.
Factors influencing lung re-expansion include the patient’s age, underlying lung conditions, type of pneumothorax, duration of lung collapse, and treatment measures. Older individuals generally experience slower lung re-expansion. Traumatic pneumothorax typically takes longer to resolve compared to closed pneumothorax. Patients with underlying lung diseases or prolonged lung collapse also experience slower recovery. Lung re-expansion takes longer with bed rest alone compared to thoracic drainage or thoracentesis. Conditions such as bronchopleural fistula, thickened visceral pleura, or bronchial obstruction can hinder lung re-expansion and may lead to chronic persistent pneumothorax.
Treatment Methods
Conservative Management
Indications for conservative management often include:
- Stable pneumothorax with a small volume of air, where lung compression is less than 20% and symptoms are mild or absent.
- First-time occurrence without significant pulmonary bullae observed on CT imaging.
- Absence of associated hemothorax or other complications.
Patients are advised to rest in bed, with observation, oxygen supplementation, and, if necessary, sedative or analgesic treatment while waiting for the air to be resorbed spontaneously.
In patients with spontaneous pneumothorax, the air resorption rate (as measured by the pneumothorax area on chest X-ray) is approximately 1.25%-2.20% per day. High-concentration oxygen therapy can increase arterial PaO2, reduce nitrogen partial pressure, and enhance the nitrogen pressure gradient between the pleural cavity and blood, thereby accelerating the resorption of pleural air and promoting lung re-expansion. Oxygen delivered via nasal cannula or face mask at a flow rate of 10 L/min can achieve satisfactory results. Patients under conservative management require close monitoring, particularly during the first 24-48 hours after pneumothorax onset. Older patients or those with underlying pulmonary diseases (e.g., COPD), who tend to have slower healing of pleural ruptures and more severe dyspnea, are generally not suitable for conservative management, even if the pneumothorax volume is small.
Air Evacuation
When lung compression exceeds 20%, particularly in patients with poor pulmonary function or underlying lung diseases, air evacuation becomes the primary approach. Tension pneumothorax and open pneumothorax require emergency air evacuation.
Thoracentesis for air evacuation is suitable for patients with 20%-50% unilateral lung compression, mild dyspnea, and preserved cardiopulmonary function in cases of closed pneumothorax. The puncture site is typically the second intercostal space at the midclavicular line on the affected side, though the fourth, fifth, or sixth intercostal spaces in the anterior axillary region can also be used. For localized pneumothorax, CT-guided puncture may be necessary. After skin disinfection, a pneumothorax needle or fine catheter is inserted into the pleural cavity, connected to a 50 mL or 100 mL syringe, and air is aspirated until dyspnea improves. The volume of air aspirated in one session should not exceed 1,000 mL. Subsequent aspirations can be performed daily or every other day depending on lung re-expansion.
In cases of tension pneumothorax, emergency decompression is critical to prevent severe complications. If thoracic catheter placement is not immediately available, a large-bore needle can be inserted into the pleural cavity to relieve pressure.
Closed thoracic drainage is indicated for unstable pneumothorax, significant dyspnea, severe lung compression, open or tension pneumothorax, and recurrent pneumothorax. Regardless of the pneumothorax volume, closed thoracic drainage should be performed promptly. Patients with secondary pneumothorax often require catheter placement, but the efficacy is generally lower than in primary pneumothorax, and repeated catheterization may be necessary for some patients.
The catheter insertion site is typically at the second intercostal space lateral to the midclavicular line or the fourth or fifth intercostal space along the anterior axillary line. For localized pneumothorax or cases requiring drainage of pleural effusion, the insertion site is determined based on imaging findings from chest X-ray or CT. After local anesthesia, a 1.5-2 cm skin incision is made parallel to the rib margin, and the pleural cavity is accessed with a trocar needle or through blunt dissection of the intercostal tissues. A sterile catheter (16-22F for most patients, 24-28F for those with bronchopleural fistula or receiving mechanical ventilation) is then inserted into the pleural cavity. The catheter is secured and connected to an underwater seal bottle with the tube submerged 1-2 cm below the water surface to maintain a pleural pressure of -1 to -2 cmH2O. Successful placement is indicated by continuous bubbling in the tube and rapid relief of dyspnea, with lung re-expansion occurring within hours to days.
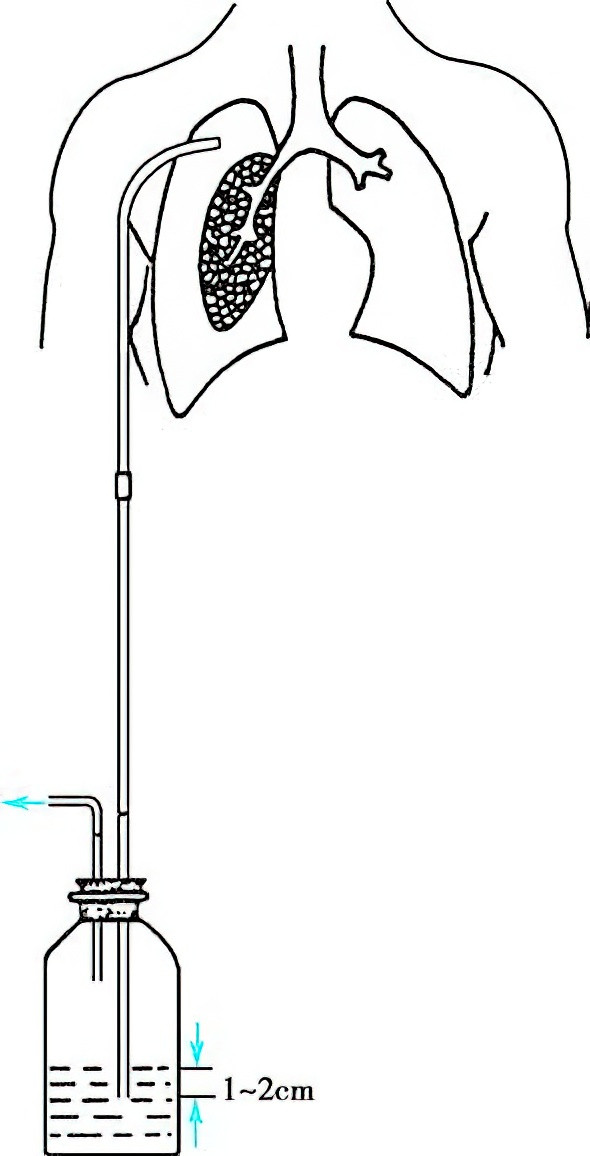
Figure 3 Water-seal closed thoracic drainage device
For patients with severe or prolonged lung compression, the drainage tube should be clamped intermittently after insertion to allow gradual drainage, thereby avoiding re-expansion pulmonary edema caused by a sudden drop in intrathoracic pressure. When no further air bubbles are observed in the drainage bottle and the patient's symptoms of dyspnea have resolved, observation for an additional 24-48 hours is recommended. If no changes occur during this period, the drainage tube can be clamped for another 24 hours. If the patient's condition remains stable, or a chest X-ray confirms complete lung re-expansion, the tube can be removed. Approximately 70% of patients achieve lung re-expansion within three days of closed drainage.
If air bubbles are still observed in the drainage bottle but the patient's symptoms show minimal improvement, the possibility of tube obstruction or partial displacement from the pleural cavity should be considered. In such cases, the tube may need to be replaced, or alternative interventions should be implemented.
In cases of primary spontaneous pneumothorax (PSP), complete lung re-expansion can often be achieved with catheter drainage. However, in secondary spontaneous pneumothorax (SSP), the presence of pleural adhesions or loculated pneumothorax may render single-tube drainage less effective, and multiple drainage tubes may need to be inserted into the affected side. For bilateral pneumothorax, simultaneous catheter drainage of both pleural cavities may be required. If the pleural rupture persists following drainage, as indicated by continuous air leakage in the drainage bottle, negative pressure suction may be applied.
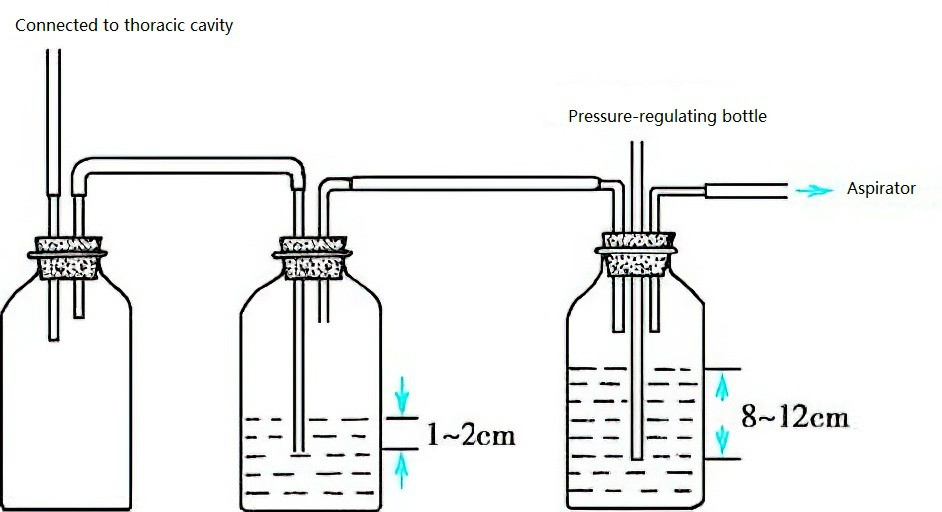
Figure 4 Negative pressure suction water bottle
Low negative pressure suction devices are preferred. If the suction pressure of the device is excessively high, a pressure-regulating bottle can be used to adjust it. The typical negative pressure setting is -10 to -20 cmH2O. If the negative pressure exceeds the set value, air enters the pressure-regulating bottle through a pressure adjustment tube, ensuring that the negative pressure applied to the pleural cavity does not exceed the set value. This prevents excessive suction from causing lung injury.
Continuous negative pressure suction is recommended. If the lung has not re-expanded in 12 hours, the underlying cause should be investigated. The absence of air bubbles indicates that the lung has re-expanded, and negative pressure suction can be discontinued. Observation for 2-3 days is suggested, and if a chest X-ray confirms no recurrence of the pneumothorax, the drainage tube can be removed.
The water-seal bottle should be placed below the level of the patient's chest (e.g., under the bed) to prevent backflow of water into the pleural cavity. During air evacuation using catheter drainage, aseptic techniques should be maintained to prevent infection.
Pleurodesis
Due to the high recurrence rate of pneumothorax, pleurodesis may be performed to prevent recurrence. This involves introducing a sclerosing agent into the pleural cavity to induce aseptic pleuritis, causing adhesion of the visceral and parietal pleura and obliteration of the pleural space. This method is suitable for patients who are not candidates for surgery or who refuse surgical intervention, including the following cases:
- Persistent or recurrent pneumothorax.
- Bilateral pneumothorax.
- Pneumothorax associated with pulmonary bullae.
- Patients with impaired pulmonary function who cannot tolerate surgery.
Commonly used sclerosing agents include talc, povidone-iodine, doxycycline, and tetracycline, which are diluted in 60-100 mL of saline and injected into the pleural cavity through a drainage tube. The tube is clamped for 1-2 hours before being reopened for drainage. Alternatively, medical talc can be sprayed directly onto the pleura under thoracoscopic guidance.
Before administering the sclerosing agent, complete lung re-expansion should be achieved via closed thoracic drainage. A local anesthetic, such as lidocaine, is injected into the pleural cavity to prevent severe localized pain caused by the sclerosing agent. The patient is then repositioned to allow the anesthetic to evenly coat the pleura. After 15-20 minutes, the sclerosing agent is injected. If the first attempt is ineffective, the procedure can be repeated. After injection, the patient is observed for 1-3 days, and the drainage tube is removed once a chest X-ray confirms resolution of the pneumothorax.
The main adverse reactions to this method include chest pain and fever. Talc can cause acute respiratory distress syndrome (ARDS), and caution is advised when using this agent.
Endobronchial Occlusion
Endobronchial occlusion involves the use of micro-balloon catheters or embolic agents to block the bronchus, leading to atelectasis of the distal lung and closure of ruptured pulmonary bullae. This procedure is typically performed while the patient undergoes intercostal catheter drainage. Following the placement of a micro-balloon (e.g., a silicone balloon), the air leakage in the water-seal bottle is monitored. If no further air bubbles are observed, the occlusion site is considered correct. After several days, the balloon can be deflated to assess for continued air leakage. If no air bubbles are observed, it indicates that the rupture has closed.
Endobronchial embolization can be performed using silicone plugs, fibrin glue, autologous blood, or other materials.
Surgical Treatment
While most patients achieve temporary resolution of pneumothorax through thoracentesis or closed thoracic drainage, more than 30% experience persistent or recurrent pneumothorax. The likelihood of recurrence increases with each subsequent episode. For recurrent spontaneous pneumothorax, surgical treatment involving resection of pulmonary bullae combined with pleurodesis is considered an effective approach.
Surgical intervention is also indicated in the following cases:
- Persistent pneumothorax.
- Hemopneumothorax.
- Bilateral pneumothorax.
- Recurrent pneumothorax.
- Tension pneumothorax unresponsive to drainage.
- Incomplete lung expansion due to pleural thickening.
- Radiological evidence of multiple pulmonary bullae.
Surgical treatment has a high success rate and a low recurrence rate.
Complications and Their Management
Pyopneumothorax may result from infections caused by Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Mycobacterium tuberculosis, or various anaerobic bacteria, often secondary to pneumonia, lung abscess, or caseous pneumonia. It can also occur as a complication of iatrogenic infections following thoracentesis or intercostal drainage catheter placement. The condition is often severe and is frequently associated with the formation of bronchopleural fistula. Pathogens can usually be identified in the purulent fluid. In addition to the administration of antibiotics, management typically involves catheter drainage and saline lavage of the pleural cavity. Surgical intervention may be considered depending on the specific clinical situation.
Hemopneumothorax, characterized by the presence of both air and blood in the pleural cavity, is often associated with the rupture of blood vessels within pleural adhesions. Hemorrhage generally stops spontaneously after complete lung re-expansion. If hemorrhage persists, treatment may include air evacuation, fluid drainage, and appropriate blood transfusion. Surgical intervention to ligate the bleeding vessel may be necessary in cases of ongoing hemorrhage.
Mediastinal emphysema and subcutaneous emphysema occur when air escapes from ruptured alveoli into the pulmonary interstitium, forming interstitial pulmonary emphysema. The air may travel along vascular sheaths into the mediastinum, and in some cases, further into the subcutaneous tissues of the neck, chest, or abdomen, resulting in subcutaneous emphysema. Subcutaneous emphysema may also occur following needle puncture or incision during air evacuation or closed thoracic drainage for tension pneumothorax, leading to localized or generalized subcutaneous emphysema and mediastinal emphysema.
Most patients do not exhibit symptoms, although subcutaneous air accumulation in the neck may cause visible thickening. Air accumulation in the mediastinum may compress major mediastinal blood vessels, leading to symptoms such as dry cough, dyspnea, emesis, and retrosternal pain, which may radiate to both shoulders or arms. The pain often worsens with respiratory movements or swallowing. Severe cases may present with cyanosis, jugular venous distension, tachycardia, hypotension, reduced or absent cardiac dullness on percussion, and muffled heart sounds. On chest X-ray, a translucent band may be observed adjacent to the mediastinum or cardiac border, predominantly on the left side.
Subcutaneous and mediastinal emphysema typically resolve spontaneously as intrapleural air is evacuated and pressure is relieved. High-concentration oxygen inhalation can increase the oxygen concentration within the mediastinum, facilitating the resolution of emphysema. If tension from mediastinal emphysema severely impacts respiration or circulation, decompression through incision at the suprasternal notch may be necessary.
Prevention
The best preventive measure is the treatment of underlying conditions. Air travel is contraindicated for pneumothorax patients until complete lung re-expansion is confirmed, typically one week after recovery. The British Thoracic Society recommends avoiding air travel for one year if surgical intervention has not been performed.