Chronic obstructive pulmonary disease (COPD) is a heterogeneous lung disease characterized by chronic respiratory symptoms (dyspnea, cough, expectoration) and persistent, progressively worsening airflow limitation caused by airway abnormalities (bronchitis, bronchiolitis) and/or alveolar abnormalities (emphysema). Irreversible airflow limitation is the key diagnostic feature of COPD. A post-bronchodilator forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) ratio (FEV1/FVC) of less than 70% indicates persistent airflow limitation.
COPD is closely associated with chronic bronchitis and emphysema. Chronic bronchitis is defined as a condition where a patient experiences cough and expectoration for more than 3 months per year, for at least 2 consecutive years, after excluding other known causes of chronic cough. Emphysema refers to abnormal, permanent enlargement of air spaces distal to the terminal bronchioles, accompanied by destruction of alveoli and bronchioles without significant pulmonary fibrosis. When patients with chronic bronchitis or emphysema exhibit persistent airflow limitation on pulmonary function tests, they can be diagnosed with COPD. However, if a patient has chronic bronchitis and/or emphysema without persistent airflow limitation, they cannot be diagnosed with COPD.
Certain diseases with known causes or characteristic pathological features can also lead to persistent airflow limitation, such as bronchiectasis, fibrotic lesions from pulmonary tuberculosis, severe interstitial lung disease, diffuse panbronchiolitis, and obliterative bronchiolitis. However, these conditions are not classified as COPD.
Etiology
The causes of COPD are similar to those of chronic bronchitis and are likely the result of prolonged interactions between environmental and individual factors.
Pathogenesis
Inflammatory Mechanisms
Chronic inflammation of the airways, lung parenchyma, and pulmonary vasculature is a hallmark of COPD. Inflammatory cells such as neutrophils, macrophages, and T lymphocytes are involved in the disease process. Activation and aggregation of neutrophils are key steps in COPD inflammation. Neutrophils release various bioactive substances, such as neutrophil elastase, which cause chronic mucus hypersecretion and destruction of lung parenchyma.
Protease-Antiprotease Imbalance Mechanism
Proteolytic enzymes can damage and destroy tissue, while antiproteases inhibit proteolytic enzymes like elastase. α1 antitrypsin (α1-AT) is the most potent antiprotease. Increased protease activity or reduced antiprotease levels can lead to tissue destruction and the development of emphysema. Inhalation of harmful gases and substances can increase protease production or activity, reduce antiprotease synthesis, or accelerate its inactivation. Oxidative stress and smoking are risk factors that reduce antiprotease activity. Congenital α1-AT deficiency is more common in individuals of Northern European ancestry.
Oxidative Stress Mechanism
Numerous studies have shown increased oxidative stress in COPD patients. Oxidative substances include superoxide anions, hydroxyl radicals, hypochlorous acid, hydrogen peroxide, and nitric oxide. These substances can directly damage biomolecules such as proteins, lipids, and nucleic acids, leading to cellular dysfunction or death. They can also disrupt the extracellular matrix, contribute to protease-antiprotease imbalance, and promote inflammatory responses by activating transcription factors like NF-κB, which regulate the transcription of inflammatory mediators such as IL-8, TNF-α, inducible nitric oxide synthase (NOS), and cyclooxygenase.
Other Mechanisms
Other factors that may contribute to the development and progression of COPD include abnormal lung development, infections, childhood asthma, autonomic dysfunction, malnutrition, and temperature changes.
Key Pathological Changes
The above mechanisms collectively result in two major pathological changes:
Small Airway Disease
This includes small airway inflammation, fibrosis, and mucus plugs, which significantly increase small airway resistance.
Emphysematous Changes
Emphysema reduces the normal tethering effect of alveoli on small airways, making them more prone to collapse. It also significantly decreases elastic recoil of the alveoli.
The combination of small airway disease and emphysematous changes leads to the characteristic persistent airflow limitation in COPD.
Pathology
The pathological changes of COPD primarily reflect those of chronic bronchitis and emphysema. The pathological changes of emphysema include lung hyperinflation and a loss of elasticity. The lungs appear grayish-white or pale, with multiple bullae of varying sizes visible on the surface. Under microscopic examination, the alveolar walls are thinned, the alveolar spaces are enlarged, ruptured, or form bullae, blood supply is reduced, and the elastic fiber network is destroyed. Based on the affected areas of the pulmonary lobules, obstructive emphysema can be classified into three types: centrilobular emphysema, panlobular emphysema, and a mixed type that lies between the two. Centrilobular emphysema is the most common.
Centrilobular emphysema is caused by inflammation of the terminal bronchioles or first-order respiratory bronchioles, leading to lumen narrowing and saccular dilatation of the distal second-order respiratory bronchioles. Its hallmark is the saccular dilatation of respiratory bronchioles located in the central zone of secondary lobules. Panlobular emphysema involves narrowing of the respiratory bronchioles, leading to expansion of the terminal lung structures, including alveolar ducts, alveolar sacs, and alveoli. Its hallmark is small emphysematous cavities distributed throughout the pulmonary lobules. Occasionally, both types coexist in the same lung, referred to as mixed emphysema, which often presents as peripheral expansion of the lung tissue on the basis of centrilobular emphysema.
Pathophysiology
The hallmark pathophysiological change in COPD is persistent airflow limitation, leading to impaired pulmonary ventilation. As the disease progresses, lung elasticity progressively decreases, alveoli enlarge continuously, and recoil is impaired. This results in an increase in residual volume (RV) and the percentage of RV in total lung capacity (TLC). As emphysema worsens, the alveoli surrounding capillaries are compressed by alveolar distension, leading to capillary regression and a significant reduction in pulmonary capillaries. This reduces blood flow between alveoli, creating alveoli that are ventilated but not perfused, which increases the physiological dead space. In some lung regions, blood perfusion is present, but alveolar ventilation is poor, preventing gas exchange and increasing functional shunting. These changes result in a ventilation-perfusion mismatch. Additionally, the loss of alveoli and capillaries reduces the surface area for gas diffusion, leading to impaired gas exchange. Ventilation and gas exchange dysfunction cause hypoxemia and carbon dioxide retention, which can lead to varying degrees of hypoxemia and hypercapnia, ultimately resulting in respiratory failure.
Clinical Manifestations
Symptoms
The onset of COPD is insidious, with a long disease course. Early stages may have no noticeable symptoms.
Persistent cough may last a lifetime as the disease progresses. Coughing is often more pronounced in the morning, with episodes of nocturnal coughing or expectoration.
Sputum is typically white, mucoid, or serous and frothy, occasionally streaked with blood. Expectoration is more prominent in the morning. During acute exacerbations, sputum volume increases and may become purulent.
Tachypnea or dyspnea initially occurs during intense physical activity and gradually worsens to the point where it is noticeable during daily activities or even at rest. Dyspnea is a hallmark symptom of COPD.
Wheezing is observed in some patients, especially in severe cases or during acute exacerbations.
In advanced stages, patients may experience weight loss, reduced appetite, and other systemic manifestations.
Signs
The anteroposterior diameter of the chest increases, the intercostal spaces widen, and the subxiphoid angle of the sternum broadens, forming a barrel chest. Some patients exhibit shallow, rapid breathing, and severe cases may show pursed-lip breathing.
Bilateral vocal fremitus is diminished.
Hyperresonant percussion sounds are heard over the lungs, cardiac dullness is reduced, and the lower lung and liver dullness borders are displaced downward.
Breath sounds are diminished bilaterally, with prolonged expiratory phases. Some patients may have crackles and/or wheezes.
Laboratory and Other Auxiliary Examinations
Pulmonary Function Tests
These are the primary methods for determining persistent airflow limitation. A post-bronchodilator FEV1/FVC < 70% confirms persistent airflow limitation. Increased TLC, functional residual capacity (FRC), and RV, along with reduced vital capacity (VC), indicate lung hyperinflation.
Chest X-ray
Early-stage COPD may show no abnormalities on chest X-rays. Over time, nonspecific changes such as thickened and disorganized pulmonary markings or emphysematous changes may appear. While X-rays have limited specificity for diagnosing COPD, they are valuable for differentiating COPD from other lung diseases and identifying complications such as spontaneous pneumothorax or pneumonia.
Chest CT Scan
CT scans can reveal small airway changes, emphysema, and complications of COPD. Their primary clinical utility lies in excluding other respiratory diseases with similar symptoms. High-resolution CT (HRCT) is particularly sensitive and specific for distinguishing centrilobular from panlobular emphysema and determining the size and number of bullae. CT scans are also valuable for assessing the potential benefits of procedures like bullectomy or lung volume reduction surgery. For smokers with COPD, low-dose CT scans are recommended for lung cancer screening.
Arterial Blood Gas Analysis
This is important for identifying hypoxemia, hypercapnia, acid-base imbalances, and determining the type of respiratory failure.
Other Tests
In cases of bacterial infections, peripheral white blood cell counts may be elevated with a left shift. Sputum cultures can help identify causative pathogens.
Diagnosis
COPD can be clinically suspected based on a history of risk factors (e.g., smoking), clinical symptoms, and signs. Pulmonary function testing to confirm persistent airflow limitation is essential for diagnosing COPD. A post-bronchodilator FEV1/FVC < 70% is the threshold for confirming persistent airflow limitation. If other known causes or diseases with characteristic pathological features of airflow limitation can be excluded, a definitive diagnosis of COPD can be established.
Assessment of Disease Severity in Stable COPD
Currently, a comprehensive index system is recommended for assessing the severity of stable COPD.
Pulmonary Function Assessment
The GOLD classification can be used. COPD patients with a post-bronchodilator FEV1/FVC < 70% can be further classified into different grades of airflow limitation severity based on the degree of FEV1 decline.
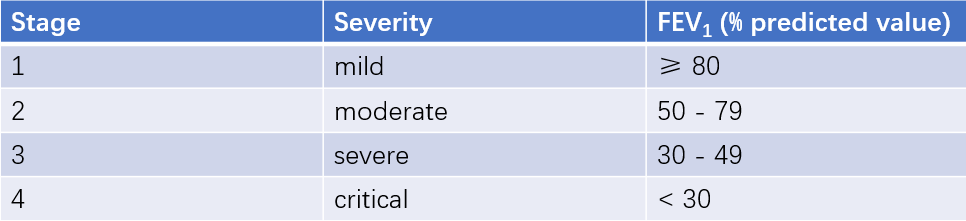
Table 1 Pulmonary function classification based on the severity of airflow limitation in COPD patients
Symptom Assessment
The modified Medical Research Council (mMRC) dyspnea scale can be used to assess the degree of dyspnea. The COPD Assessment Test (CAT) questionnaire can be used to evaluate the health impact of COPD on patients.

Table 2 mMRC questionnaire
Acute Exacerbations Risk Assessment
Two or more moderate exacerbations or at least one exacerbation requiring hospitalization in the past year indicate an increased risk of future exacerbations.
Based on symptoms, risk of exacerbations, and changes in lung function, a comprehensive assessment of disease severity in stable COPD can be made. This assessment guides the selection of primary treatment medications for stable COPD. Peripheral blood eosinophil count may have value in predicting the risk of acute exacerbations and the preventive effect of inhaled corticosteroids (ICS) on exacerbations.
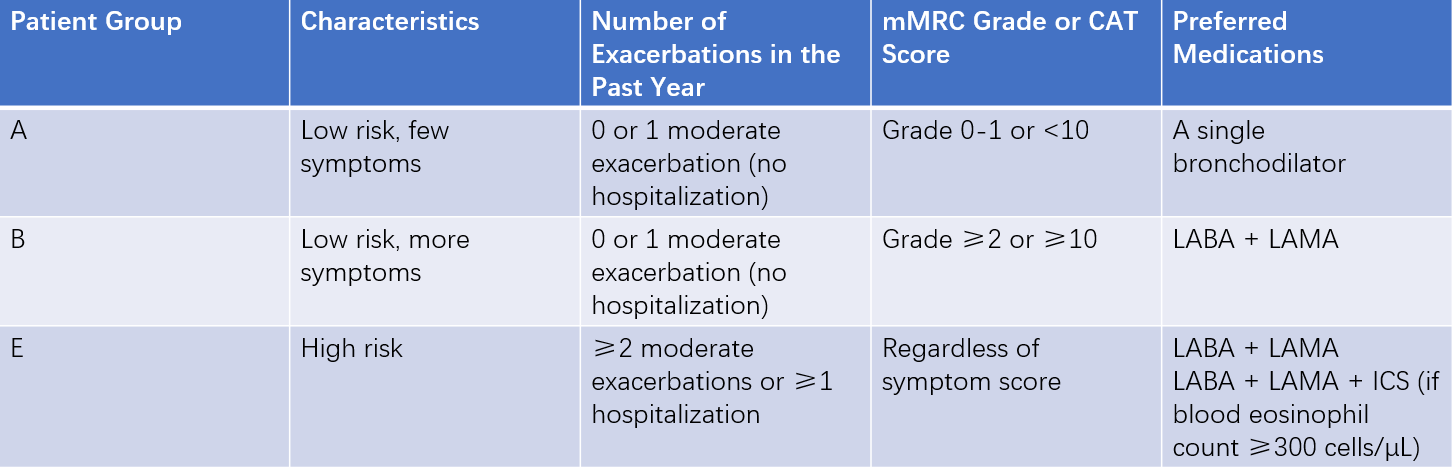
Table 3 Comprehensive assessment of COPD severity during the stable phase and recommended medications
Note: Patients with low symptoms but high risk (formerly Group C) and those with high symptoms and high risk (formerly Group D) are now combined into Group E to emphasize the clinical relevance of high exacerbation risk.
Abbreviations: LABA = Long-acting β2-agonist; LAMA = Long-acting muscarinic antagonist; ICS = Inhaled corticosteroid.
When conducting a comprehensive assessment of disease severity in COPD patients, attention should also be given to systemic comorbidities, such as cardiovascular diseases, osteoporosis, anxiety and depression, lung cancer, infections, metabolic syndrome, and diabetes, which should be addressed during treatment.
Assessment of Disease Severity in Acute Exacerbations
Acute exacerbations of COPD are defined as events characterized by increased dyspnea and/or cough and expectoration over 14 days, often accompanied by tachypnea and/or tachycardia. These events are usually associated with increased local and systemic inflammation caused by infections, pollution, or other airway-damaging factors.
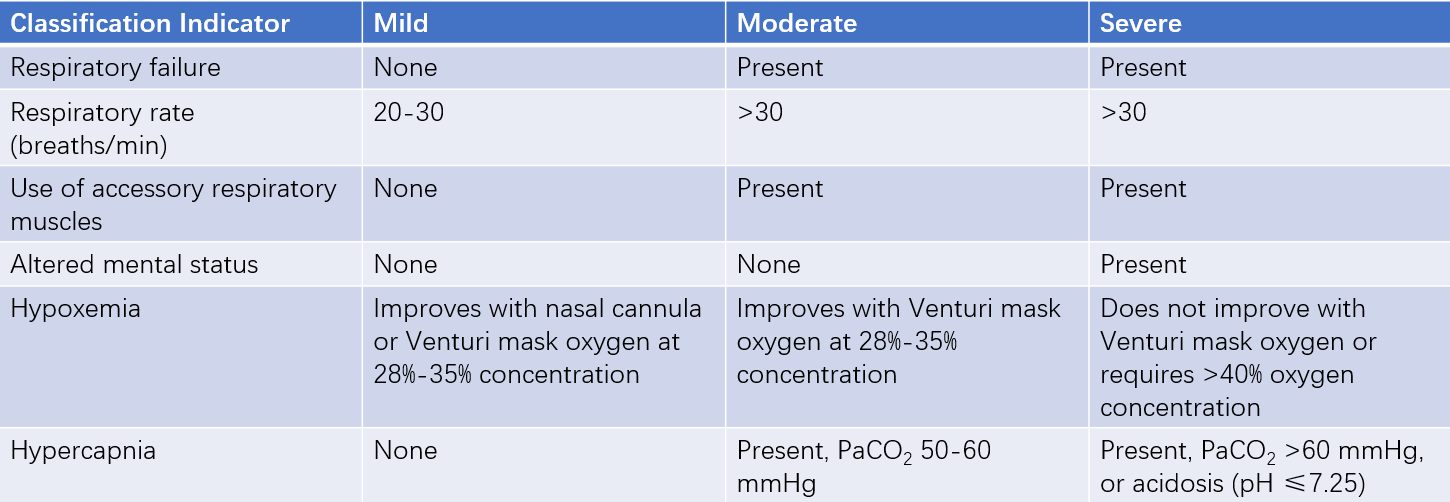
Table 4 Clinical classification of COPD acute exacerbations
Differential Diagnosis
Asthma
COPD typically has an insidious onset in middle-aged individuals, with symptoms progressing slowly and a history of long-term smoking. Asthma, on the other hand, often begins in childhood or adolescence, has fluctuating symptoms, and is commonly associated with a history of allergies, rhinitis, and/or eczema. Some patients may have a family history of asthma. Most asthma patients exhibit significant reversibility of airflow limitation, and the disease can often be effectively controlled with appropriate inhaled corticosteroids. This is a key distinguishing feature from COPD. However, some patients with long-standing asthma may develop airway remodeling, reducing the reversibility of airflow limitation, making differentiation more difficult. In such cases, a comprehensive analysis of clinical and laboratory findings is necessary. In a small subset of patients, both diseases may overlap.
Other Diseases Causing Chronic Cough and Expectoration
These include bronchiectasis, tuberculosis, lung cancer, idiopathic pulmonary fibrosis, and diffuse panbronchiolitis.
Other Diseases Causing Exertional Dyspnea
These include coronary artery disease, hypertensive heart disease, and valvular heart diseases.
Other Causes of Enlarged Air Spaces
Uniform and regular enlargement of air spaces without alveolar wall destruction does not meet the strict definition of emphysema but is often clinically referred to as emphysema, such as compensatory emphysema and senile emphysema. Clinical manifestations may include exertional dyspnea and signs of emphysema. A comprehensive analysis of clinical data is required for differentiation.
Complications
Chronic Respiratory Failure
This often occurs during acute exacerbations of COPD, with symptoms significantly worsening. Hypoxemia and/or hypercapnia develop, leading to clinical manifestations of oxygen deficiency and carbon dioxide retention.
Spontaneous Pneumothorax
If there is a sudden worsening of dyspnea accompanied by significant cyanosis, hyperresonance on percussion of the affected lung, and diminished or absent breath sounds on auscultation, spontaneous pneumothorax should be suspected. Diagnosis can be confirmed via chest X-ray.
Chronic Cor Pulmonale
COPD can lead to a reduction in the pulmonary vascular bed and hypoxia-induced pulmonary artery constriction and vascular remodeling, resulting in pulmonary hypertension, right ventricular hypertrophy and dilation, and eventually right heart failure.
Treatment
Treatment During the Stable Phase
Education and Management
The most critical aspect is encouraging smoking cessation in patients who smoke, as this is the most effective measure to slow the decline in lung function, though it is also the most challenging to implement. Various educational measures should be employed for smokers, and pharmacological aids can be considered if available. For patients exposed to occupational or environmental dust and irritant gases, removing them from the polluted environment is essential.
Bronchodilators
Bronchodilators are the primary treatment for symptom control. Selection should be based on the severity of the patient's condition and their response to medication. Combining bronchodilators with different mechanisms of action can enhance the bronchodilatory effect.
β2-adrenergic receptor agonists:
- Short-acting formulations: Salbutamol inhaler, 100-200 μg (1-2 puffs) per dose, with effects lasting 4-6 hours. Do not exceed 8-12 puffs per 24 hours.
- Long-acting formulations: Salmeterol and formoterol are inhaled twice daily, while indacaterol and vilanterol are inhaled once daily.
Anticholinergics:
- Short-acting formulations: Ipratropium bromide inhaler, 40-80 μg per dose (20 μg per puff), with effects lasting 6-8 hours, administered 3-4 times daily.
- Long-acting formulations: Tiotropium bromide, glycopyrronium bromide, and umeclidinium bromide are inhaled once daily.
Methylxanthines:
- Sustained-release or controlled-release theophylline tablets, 0.2 g, taken once every 12 hours.
- Aminophylline, 0.1 g, taken three times daily.
- Inhaled Corticosteroids (ICS)
For some patients whose symptoms are not controlled despite adequate maintenance therapy with long-acting bronchodilators, ICS can be considered as an add-on treatment. Triple therapy with two bronchodilators and an ICS is commonly used.
Indications for ICS use include:
- A history of hospitalization due to COPD exacerbations.
- Two or more moderate exacerbations per year.
- Peripheral blood eosinophil counts ≥300 cells/μL.
- A history of asthma or asthma-like features.
For patients with one moderate exacerbation per year and eosinophil counts between 100-300 cells/μL, ICS can be added to long-acting bronchodilators. However, ICS is not recommended for patients with eosinophil counts <100 cells/μL, recurrent pneumonia, or concomitant mycobacterial infections. Commonly used ICS include budesonide, fluticasone, and beclomethasone.
Expectorants can be used for patients with difficulty clearing sputum. Common medications include:
- Ambroxol hydrochloride, 30 mg, three times daily.
- N-acetylcysteine, 0.6 g, twice daily.
- Carbocisteine, 0.5 g, three times daily.
The latter two medications may reduce the risk of acute exacerbations in some patients.
Other medications:
- Phosphodiesterase-4 Inhibitors: Roflumilast can be used in patients with a history of frequent COPD exacerbations to reduce the risk of exacerbations.
- Macrolides: Studies have shown that long-term use of macrolides (e.g., erythromycin or azithromycin) for one year can reduce the frequency of exacerbations in certain patients with frequent exacerbations. However, this may lead to bacterial resistance and hearing loss.
- Long-term oxygen therapy (LTOT): LTOT can improve the quality of life and survival in COPD patients with chronic respiratory failure. It also has beneficial effects on hemodynamics, exercise capacity, and mental health.
Indications for LTOT include:
- PaO2 ≤ 55 mmHg or SaO2 ≤ 88%, with or without hypercapnia.
- PaO2 between 55-60 mmHg or SaO2 < 89%, accompanied by pulmonary hypertension, right heart failure, or polycythemia (hematocrit > 0.55).
Oxygen is typically delivered via nasal cannula at a flow rate of 1.0-2.0 L/min for more than 15 hours per day. The goal is to achieve a PaO2 ≥ 60 mmHg and/or an SaO2 ≥ 90% at sea level under resting conditions.
Rehabilitation therapy is an essential treatment for stable COPD patients, particularly those with limited physical activity due to progressive airflow limitation and severe dyspnea. It aims to improve exercise capacity and quality of life. Rehabilitation includes various measures such as respiratory physiotherapy, muscle training, nutritional support, psychological therapy, and patient education.
Treatment During Acute Exacerbations
The most common causes of acute exacerbations are bacterial or viral infections. The severity of the exacerbation should be assessed to determine whether outpatient or inpatient treatment is required.
The medications used are similar to those in the stable phase. For patients with severe wheezing symptoms, nebulized therapy with higher doses can be administered, such as salbutamol 500 μg or a combination of salbutamol 1,000 μg and ipratropium bromide 250-500 μg, delivered via a small nebulizer to relieve symptoms.
For patients with hypoxemia, oxygen can be delivered via nasal cannula or a Venturi mask. When using a nasal cannula, the inhaled oxygen concentration should be 28-30%. Care must be taken to avoid excessive oxygen concentrations, which may lead to carbon dioxide retention.
Antibiotics should be used in patients with worsening dyspnea, increased sputum production, or purulent sputum. The choice of antibiotic should be based on the common pathogens in the patient’s location and their antibiotic susceptibility.
- Outpatient treatment: Amoxicillin/clavulanate, cefixime, cefuroxime, levofloxacin, or moxifloxacin can be administered orally.
- Severe cases: Third-generation cephalosporins such as ceftriaxone (2.0 g in normal saline, given intravenously once daily) can be used.
- Inpatient treatment: Antibiotics should be selected based on suspected pathogens and local antibiotic resistance patterns. Common choices include a combination of β-lactam/β-lactamase inhibitors with macrolides or respiratory fluoroquinolones, typically administered intravenously. If a specific pathogen is identified, antibiotic selection should be guided by sensitivity testing.
For patients requiring hospitalization during acute exacerbations, prednisone 30-40 mg/day can be administered orally. Alternatively, methylprednisolone 40-80 mg can be given intravenously once daily for 5 consecutive days.
Mechanical ventilation may be necessary for patients with severe respiratory failure.
Adequate fluid and electrolyte supplementation should be provided to maintain water-electrolyte balance.
Nutritional support should be adjusted based on the patient’s gastrointestinal function to ensure adequate caloric, protein, and vitamin intake. Parenteral nutrition may be considered if necessary.
Effective sputum clearance is critical. The most effective approach is ensuring adequate hydration to thin the mucus. Other methods include stimulating coughing, chest percussion, and postural drainage.
Comorbidities (e.g., coronary artery disease, diabetes) and complications (e.g., spontaneous pneumothorax, shock, disseminated intravascular coagulation, upper gastrointestinal bleeding, renal failure) should be actively managed.
Interventional Therapy
Interventional therapy for COPD primarily involves bronchoscopic interventions, which aim to reduce lung hyperinflation in patients with severe emphysema. Common bronchoscopic procedures include:
- Endobronchial one-way valves
- Airway bypass stents
- Lung volume reduction via bronchial occlusion
- Thermal vapor ablation
- Coil-based lung volume reduction
The choice of procedure should be guided by clinical and imaging findings.
Surgical Treatment
Surgical interventions are only suitable for a small subset of patients with specific indications. Proper patient selection can lead to improvements in lung function and relief of dyspnea. However, due to the high surgical risks and costs, surgical options must be carefully considered.
Surgical methods include:
- Bullectomy: Removal of large bullae.
- Lung volume reduction surgery (LVRS): To improve lung mechanics.
- Lung transplantation: This provides a potential treatment option for patients with end-stage COPD. However, it is associated with high technical demands, limited donor availability, and significant costs.
Prevention
COPD leads to irreversible airflow limitation, and once the disease develops, treatment outcomes are often suboptimal. A comprehensive approach following the principles of promotion, prevention, diagnosis, control, treatment, and rehabilitation is essential, with a focus on prevention.
Smoking Cessation
Smoking cessation is the most important preventive measure for COPD. Quitting smoking at any stage of the disease helps prevent the onset and progression of COPD.
Environmental Control
Reducing exposure to harmful gases or particulate matter and controlling environmental pollution are critical.
Prevention of Respiratory Infections
Actively preventing and treating respiratory infections during infancy and childhood is important. Vaccinations, such as influenza vaccines, pneumococcal vaccines, bacterial lysates, and BCG polysaccharide nucleic acid, may help prevent recurrent infections in COPD patients.
Physical Activity
Regular exercise and physical fitness can enhance immunity and reduce the risk of COPD.
Early Detection
For individuals with high-risk factors for COPD, regular pulmonary function monitoring is recommended to enable early detection and timely intervention.