Pneumonia refers to inflammation of the terminal airways, alveoli, and pulmonary interstitium, which can be caused by pathogenic microorganisms, physical or chemical factors, immune damage, allergies, or medications. Bacterial pneumonia is the most common form of pneumonia and one of the most prevalent infectious diseases. Before the advent of antibiotics, bacterial pneumonia posed a significant threat to the health of children and older adults. The introduction and development of antibiotics initially led to a marked reduction in pneumonia mortality. However, in recent years, despite the use of potent antibiotics and effective vaccines, the mortality rate of pneumonia has not further declined and has even shown an upward trend.
Epidemiology
The annual incidence of community-acquired pneumonia (CAP) and hospital-acquired pneumonia (HAP) is approximately 5-11 per 1,000 persons and 5-10 per 1,000 hospitalized patients, respectively. The mortality rate for CAP patients treated on an outpatient basis is less than 1%-5%, while it averages 12% for hospitalized patients and reaches approximately 40% for those admitted to intensive care units (ICUs). The mortality rate associated with HAP ranges from 15.5% to 38.2%. The high incidence and mortality rates are linked to factors such as an aging population, smoking, underlying diseases, and weakened immune function, including chronic obstructive pulmonary disease (COPD), heart failure, cancer, diabetes, uremia, neurological disorders, drug addiction, alcoholism, AIDS, prolonged illness, major surgeries, use of immunosuppressants, and organ transplantation. Additionally, changes in pathogens, the emergence of new pathogens, difficulties in pathogen diagnosis, and the irrational use of antibiotics leading to increased bacterial resistance—particularly multidrug-resistant (MDR) pathogens—also contribute to these trends.
Etiology, Pathogenesis, and Pathology
The normal respiratory immune defense mechanisms (e.g., the bronchial mucociliary clearance system and the integrity of alveolar macrophages) protect the lower respiratory tract from infection by bacteria and other pathogens. The occurrence of pneumonia depends on two factors: the pathogen and the host. Pneumonia develops when the pathogen is abundant or highly virulent and/or when the host's local or systemic respiratory immune defenses are impaired. Pathogens can cause CAP through the following pathways:
- Inhalation of airborne pathogens.
- Hematogenous dissemination.
- Spread from adjacent infected sites.
- Aspiration of colonized bacteria from the upper respiratory tract.
HAP is more commonly caused by aspiration of colonized bacteria from the gastrointestinal tract (e.g., gastroesophageal reflux) and/or inhalation of environmental pathogens through artificial airways. Once pathogens reach the lower respiratory tract, they proliferate, leading to capillary congestion, edema, fibrin exudation, and cellular infiltration in the alveoli. Except for pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella pneumoniae, which can cause necrotizing lesions and cavitation in the lung tissue, most cases of pneumonia resolve without leaving scars, with the lung's structure and function fully recovering.
Classification
Pneumonia can be classified based on anatomical features, etiology, or the environment in which it occurs.
Anatomical Classification
Lobar (Alveolar) Pneumonia
Pathogens initially cause inflammation in the alveoli, spreading to other alveoli via the alveolar pores (Cohn's pores), leading to inflammation in part or all of a lung segment or lobe. This type typically presents as parenchymal inflammation without involving the bronchi and is most commonly caused by Streptococcus pneumoniae. Imaging shows consolidation in the affected lobe or segment.
Lobular (Bronchial) Pneumonia
Pathogens invade via the bronchi, causing inflammation in the bronchioles, terminal bronchioles, and alveoli. It often occurs secondary to other conditions such as bronchitis, bronchiectasis, upper respiratory viral infections, or prolonged bed rest in critically ill patients. Common pathogens include Streptococcus pneumoniae, Staphylococcus aureus, viruses, Mycoplasma pneumoniae, and Legionella. Imaging shows irregular patchy opacities distributed along the pulmonary markings, with blurred and hazy edges, often involving the lower lobes without signs of consolidation.
Interstitial Pneumonia
This type primarily involves inflammation of the pulmonary interstitium, including the bronchial walls, peribronchial tissues, and alveolar walls. Since the lesions are confined to the interstitium, respiratory symptoms are relatively mild, but extensive lesions can cause significant dyspnea. It can be caused by bacteria, Mycoplasma pneumoniae, Chlamydia pneumoniae, viruses, or Pneumocystis jirovecii. Imaging shows irregular opacities in one or both lower lung fields, which may appear as ground-glass opacities, reticular patterns, or small areas of atelectasis.
Etiological Classification
Bacterial Pneumonia
This is caused by pathogens such as Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus pyogenes, Klebsiella pneumoniae, Haemophilus influenzae, Pseudomonas aeruginosa, and Acinetobacter baumannii.
Atypical Pneumonia
This is caused by atypical pathogens such as Legionella, Mycoplasma pneumoniae, and Chlamydia pneumoniae.
Viral Pneumonia
This is caused by viruses such as coronaviruses, adenoviruses, respiratory syncytial virus, influenza viruses, measles virus, cytomegalovirus, and herpes simplex virus.
Fungal Pneumonia
This is caused by fungi such as Candida, Aspergillus, Cryptococcus, Pneumocystis jirovecii, and Mucor species.
Other Pathogens
These include infections caused by Rickettsia (e.g., Coxiella burnetii in Q fever), Toxoplasma (e.g., Toxoplasma gondii), and parasites (e.g., Echinococcus, Paragonimus, Schistosoma).
Pneumonia Caused by Physical or Chemical Factors
This includes radiation-induced pneumonia, chemical pneumonia caused by aspiration of gastric acid, and lipoid pneumonia resulting from inflammatory reactions to inhaled or endogenous lipid substances. Pneumonia caused by physical or chemical factors is generally excluded from the conventional definition of pneumonia.
Environmental Classification
Due to the low positive rate of bacterial tests and the delayed results of cultures, etiological classification is often difficult in clinical practice. Currently, pneumonia is commonly classified based on the environment in which it is acquired, as the pathogens involved vary depending on the setting, which aids in guiding empirical treatment.
Community-acquired Pneumonia (CAP)
This refers to infectious inflammation of the lung parenchyma (including alveolar walls, i.e., the pulmonary interstitium) acquired outside of a hospital. CAP also includes cases where pathogens with a known incubation period cause symptoms during this period after hospital admission. The clinical diagnostic criteria are:
- Onset in the community.
- Clinical manifestations related to pneumonia:
- New-onset cough, sputum production, or worsening of pre-existing respiratory symptoms with purulent sputum, with or without chest pain, dyspnea, or hemoptysis.
- Fever.
- Signs of lung consolidation and/or audible crackles.
- White blood cell count >10×109 /L or <4×109 /L, with or without a left shift in neutrophils.
- Chest imaging showing patchy or interstitial infiltrative opacities, with or without pleural effusion.
A clinical diagnosis can be established if criteria (1) and (3) are met along with any one of the criteria under (2), excluding other conditions such as tuberculosis, lung tumors, non-infectious interstitial lung diseases, pulmonary edema, atelectasis, pulmonary embolism, eosinophilic pneumonia, and pulmonary vasculitis. Common pathogens include Streptococcus pneumoniae, Mycoplasma pneumoniae, Chlamydia pneumoniae, Haemophilus influenzae, and respiratory viruses (e.g., influenza A and B, adenovirus, respiratory syncytial virus, parainfluenza virus).
Hospital-acquired Pneumonia (HAP)
This refers to pneumonia that develops 48 hours or more after hospital admission in patients not on invasive mechanical ventilation and not in the incubation period of a pathogen upon admission. Ventilator-associated pneumonia (VAP) refers to pneumonia occurring 48 hours after mechanical ventilation in patients with endotracheal intubation or tracheostomy, or within 48 hours after extubation. Imaging shows new or progressive infiltrates, consolidations, or ground-glass opacities. A clinical diagnosis requires at least two of the following three symptoms:
- Fever >38°C.
- Purulent airway secretions.
- Peripheral white blood cell count >10×109/L or <4×109/L.
The more clinical criteria met, the higher the diagnostic accuracy. Given the low specificity of clinical, laboratory, and imaging findings, HAP should be differentiated from atelectasis, heart failure with pulmonary edema, underlying lung disease, drug-induced lung injury, pulmonary embolism, and acute respiratory distress syndrome (ARDS). Following a clinical diagnosis of HAP/VAP, microbiological tests should be performed. In non-immunocompromised patients, HAP/VAP is usually caused by bacterial infections. The distribution and resistance profiles of common pathogens vary with region, hospital level, patient population, and antibiotic exposure, and they change over time. Common pathogens include Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, and Staphylococcus aureus. It is crucial to consider local hospital pathogen surveillance data when initiating empirical treatment, tailoring antibiotic selection to the pathogen spectrum and resistance patterns of the specific region, hospital, or department, as well as individual patient factors.
Clinical Manifestations
The symptoms of bacterial pneumonia can range from mild to severe, depending on the pathogen and the host's condition. Common symptoms include cough, expectoration, or exacerbation of pre-existing respiratory symptoms, often accompanied by purulent or blood-streaked sputum, with or without chest pain. Patients with extensive lesions may experience dyspnea or respiratory distress. Most patients present with fever. Early pulmonary signs may be absent, but severe cases can exhibit increased respiratory rate, nasal flaring, and cyanosis. Typical signs of lung consolidation include dullness to percussion, increased vocal fremitus, and bronchial breath sounds, as well as crackles on auscultation. In cases with pleural effusion, the affected side may show dullness to percussion, decreased vocal fremitus, and diminished breath sounds.
Diagnosis and Differential Diagnosis
The diagnostic process for pneumonia is as follows:
- Confirming the diagnosis of pneumonia
- Assessing severity
- Identifying the pathogen
Confirming the Diagnosis of Pneumonia
Pneumonia is diagnosed based on the previously mentioned diagnostic criteria and classified as either community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP). At the same time, it is necessary to differentiate pneumonia from the following conditions:
Acute Upper Respiratory Tract Infections and Tracheobronchitis
These conditions often present with cough, expectoration, and fever but lack parenchymal infiltrates. Chest X-rays can help distinguish these conditions.
Diseases Resembling Pneumonia
Pulmonary tuberculosis often presents with systemic symptoms such as low-grade afternoon fever, diaphoresis, fatigue, weight loss, insomnia, and palpitations. Female patients may experience menstrual irregularities or amenorrhea. Chest X-rays typically show lesions in the lung apex or above the clavicle with inhomogeneous density, slow resolution, and possible cavitation or pulmonary dissemination. Mycobacterium tuberculosis can be detected in sputum. Antibiotic treatment is generally ineffective.
Lung cancer often lacks acute infectious symptoms. Hemoptysis may occur, and white blood cell counts are usually normal. However, lung cancer can be complicated by obstructive pneumonia, where the tumor opacity becomes more apparent after inflammation subsides with antibiotic treatment. Enlarged hilar lymph nodes or atelectasis may also be observed. If pneumonia does not resolve after antibiotic treatment or recurs in the same location, close follow-up is necessary. For older patients with a history of smoking, further diagnostic tests such as CT, MRI, bronchoscopy, and sputum cytology may be needed to avoid missed diagnoses.
Pulmonary embolism is often associated with risk factors for venous thrombosis, such as thrombotic phlebitis, cardiovascular or pulmonary diseases, trauma, surgery, or cancer. Symptoms include significant dyspnea, hemoptysis, and syncope. Chest X-rays may show regional reductions in pulmonary vascular markings or wedgy opacities pointing toward the hilum. Arterial blood gas analysis often reveals hypoxemia and hypocapnia. Tests such as D-dimer, CT pulmonary angiography, radionuclide ventilation/perfusion scanning, and MRI can aid in diagnosis.
Non-infectious pulmonary infiltrates such as interstitial pneumonia, pulmonary edema, atelectasis, and pulmonary vasculitis should also be considered.
Assessing Severity
Once a pneumonia diagnosis is established, assessing the severity of the disease is critical for determining whether the patient requires outpatient, inpatient, or ICU treatment. The severity of pneumonia depends on three main factors: the extent of local pulmonary inflammation, the dissemination of inflammation, and the degree of systemic inflammatory response.
There are currently no universally accepted diagnostic criteria for severe pneumonia. Severe pneumonia is generally considered when patients require respiratory support (e.g., for acute respiratory failure, severe gas exchange impairment with hypercapnia, or persistent hypoxemia), circulatory support (e.g., hemodynamic instability or inadequate peripheral perfusion), and intensive monitoring and treatment.
Many countries have developed diagnostic criteria for severe pneumonia, which, although varying, emphasize the extent of lung lesions, organ perfusion, and oxygenation status. The CURB-65 score is recommended for evaluating whether CAP patients require hospitalization. CURB-65 consists of five criteria, each scoring 1 point:
- Confusion (C)
- Uremia (U): Blood urea nitrogen >7 mmol/L
- Respiratory rate (R): ≥30 breaths/min
- Blood pressure (B): Systolic <90 mmHg or diastolic ≤60 mmHg
- Age ≥65 years
A score of 0-1 suggests outpatient treatment is sufficient. A score of 2 suggests hospitalization or close outpatient monitoring. A score of 3-5 indicates hospitalization, with ICU admission considered if necessary.
Severe CAP can be diagnosed if one major criterion or three or more minor criteria are met. Such patients require close monitoring and aggressive treatment, with ICU admission if possible.
Major criteria:
- Need for mechanical ventilation via endotracheal intubation.
- Septic shock requiring vasopressors despite aggressive fluid resuscitation.
Minor criteria:
- Respiratory rate ≥30 breaths/min.
- PaO2/FiO2 ≤250 mmHg (1 mmHg = 0.133 kPa).
- Multilobar infiltrates.
- Altered mental status or disorientation.
- Blood urea nitrogen ≥7.14 mmol/L (20 mg/dL).
- Systolic blood pressure <90 mmHg requiring aggressive fluid resuscitation.
Identifying the Pathogen
Since the upper respiratory tract contains many microorganisms as part of its normal flora, lower respiratory tract secretions or sputum passing through the oropharynx are easily contaminated. In patients with chronic airway diseases, older individuals, or critically ill patients, colonization of the respiratory tract is significantly increased, complicating the isolation and identification of pathogens in sputum. Additionally, prior antibiotic use can affect bacterial culture results. Therefore, respiratory specimens should ideally be collected before antibiotic administration, avoiding contamination, and sent for testing promptly.
Sputum
Sputum collection is convenient and the most commonly used method for obtaining lower respiratory tract specimens. Specimens should be sent for testing within 2 hours at room temperature. A direct smear is examined under a microscope to assess cell composition. Specimens with <10 squamous epithelial cells and >25 white blood cells per low-power field, or a squamous epithelial cell-to-white blood cell ratio <1:2.5, are considered qualified and relatively uncontaminated for culture. Pathogenic or opportunistic bacteria isolated in concentrations ≥107 cfu/mL are considered causative pathogens, while concentrations ≤104 cfu/mL suggest contamination. Intermediate concentrations warrant repeat culture.
Bronchoscopy or Suctioning via Artificial Airways
These methods reduce contamination from oropharyngeal bacteria. Bacterial concentrations ≥105 cfu/mL in aspirates are considered pathogenic, while lower concentrations are likely contaminants.
Protected Specimen Brush
Bacterial concentrations ≥103 cfu/mL are considered pathogenic.
Bronchoalveolar Lavage (BAL)
Bacterial concentrations ≥104 cfu/mL in BAL fluid or ≥103 cfu/mL in protected BAL specimens are considered pathogenic.
Percutaneous Fine-needle Aspiration and Open Lung Biopsy
These methods have high sensitivity and specificity but are invasive and may cause complications such as pneumothorax or hemorrhage. They are generally reserved for cases unresponsive to empirical antibiotic therapy or when other diagnostic methods fail.
Blood and Pleural Fluid Cultures
Identical bacteria isolated from blood and sputum cultures can confirm the causative pathogen. Bacteria isolated from pleural fluid cultures are almost always pathogenic. Results must exclude contamination from skin flora during sample collection.
Urinary Antigen Tests
These include tests for Legionella and Streptococcus pneumoniae antigens.
Serological Tests
Specific IgM antibody titers can be measured. A fourfold increase in titers between the acute and convalescent phases confirms the diagnosis (e.g., Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella pneumophila, or viral infections). These are usually retrospective diagnoses.
Molecular Diagnostic Techniques
Nucleic acid detection methods include real-time PCR, digital PCR, isothermal amplification, and point-of-care nucleic acid testing. Real-time PCR is suitable for outpatient and inpatient screening, while digital PCR quantifies pathogen loads in respiratory specimens. Metagenomic next-generation sequencing (mNGS) is used in cases of severe respiratory infections, suspected rare pathogens, or outbreaks, especially when conventional microbiological methods fail to identify pathogens.
Despite the availability of numerous diagnostic methods, the causative pathogen remains unidentified in 40%-50% of pneumonia cases. Low pathogen detection rates and delays in microbiological and serological results mean that most pneumonia treatments, particularly initial antibiotic therapy, are empirical. However, for HAP, pneumonia in immunosuppressed hosts, and severe pneumonia unresponsive to treatment, aggressive efforts to identify the pathogen are essential to guide clinical antibiotic therapy. Clinical and radiological features can provide clues to the likely pathogen.
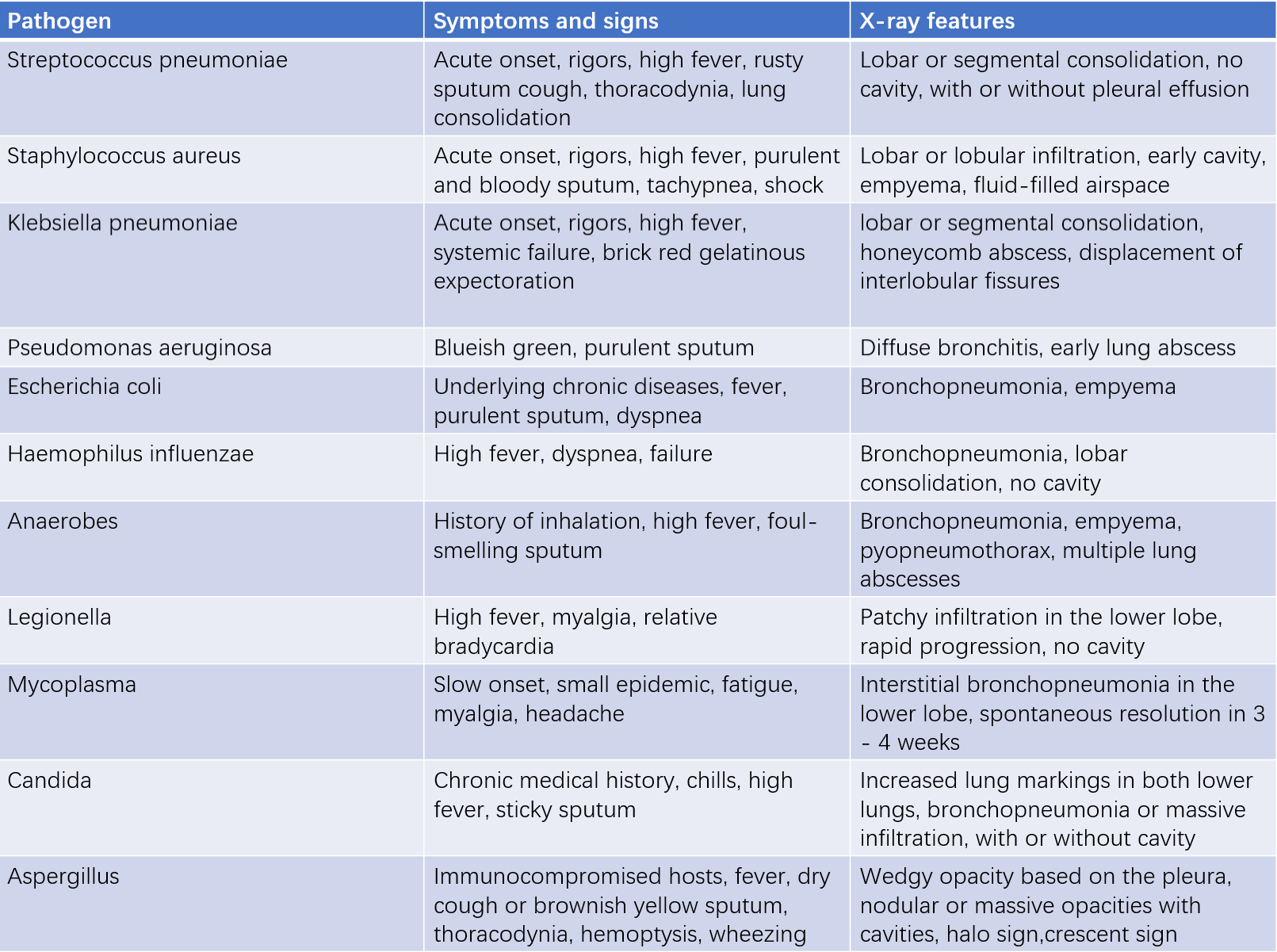
Table 1 Common symptoms, signs, and X-ray features of pneumonia
Treatment
Antimicrobial therapy is the cornerstone of pneumonia treatment, including both empirical therapy and pathogen-directed therapy. Empirical therapy is primarily based on the epidemiological data of pneumonia pathogens in the local region or institution, selecting antibiotics that likely cover the causative pathogens. Pathogen-directed therapy, on the other hand, is guided by microbiological culture results, histopathological findings, or drug susceptibility testing, selecting antibiotics to which the pathogen is sensitive in vitro. Additionally, the choice of antibiotics and administration routes should consider factors such as the patient’s age, underlying conditions, risk of aspiration, hospitalization setting (general ward vs. ICU), duration of hospitalization, and the severity of pneumonia.
For young and otherwise healthy patients with community-acquired pneumonia (CAP), commonly used antibiotics include penicillins or first-generation cephalosporins. Due to the high resistance rates of Streptococcus pneumoniae to macrolides, macrolides alone are not recommended for treating pneumonia caused by this pathogen. For drug-resistant Streptococcus pneumoniae, respiratory fluoroquinolones (e.g., moxifloxacin, gemifloxacin, or levofloxacin) can be used. For older patients, those with underlying conditions, or hospitalized CAP patients, commonly used antibiotics include respiratory fluoroquinolones, second- or third-generation cephalosporins, β-lactam/β-lactamase inhibitors, or ertapenem, which can be combined with macrolides. For hospital-acquired pneumonia (HAP), second- or third-generation cephalosporins, β-lactam/β-lactamase inhibitors, fluoroquinolones, or carbapenems are commonly used.
For severe pneumonia, broad-spectrum and potent antibiotics should be chosen as first-line therapy, administered in adequate doses and often in combination. Inadequate or inappropriate initial empirical therapy, or delayed adjustment of antibiotics based on pathogen identification, is associated with significantly higher mortality compared to correct initial treatment. For severe CAP, β-lactams combined with macrolides or fluoroquinolones are commonly used; for penicillin-allergic patients, respiratory fluoroquinolones combined with aztreonam can be used. For HAP, antibiotics with anti-Pseudomonas activity, such as β-lactams, broad-spectrum penicillins/β-lactamase inhibitors, or carbapenems, can be combined with respiratory fluoroquinolones or aminoglycosides. If multidrug-resistant (MDR) gram-positive cocci are suspected, vancomycin, teicoplanin, or linezolid can be added.
Antibiotic therapy should be initiated as early as possible. Once pneumonia is suspected, the first dose of antibiotics should be administered immediately, as earlier treatment is associated with better outcomes. After clinical stabilization, intravenous antibiotics can be switched to oral formulations. Antimicrobial therapy can generally be discontinued 2-3 days after fever resolution and significant improvement in major respiratory symptoms. However, the duration of therapy should depend on the severity of the disease, the speed of recovery, the presence of complications, and the specific pathogen, rather than the resolution of radiographic abnormalities. For mild to moderate CAP, the typical treatment duration is 5-7 days. For severe cases or those with extrapulmonary complications, the duration can be extended as needed. For atypical pathogens with slower treatment responses, therapy may be extended to 10-14 days. For pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella species, or anaerobes, which are prone to causing lung tissue necrosis, the treatment duration may be extended to 14-21 days.
Most CAP patients show clinical improvement within 72 hours after initial treatment, with symptoms such as fever reduction, improvement in respiratory symptoms, stabilization of clinical status, and gradual normalization of white blood cell counts, C-reactive protein (CRP), and procalcitonin levels. However, radiographic improvement often lags behind clinical recovery. A reassessment of the patient’s condition should be performed 72 hours after initiating treatment. For some patients who respond more slowly to treatment, as long as there is no clinical deterioration, continued observation is acceptable without rushing to change antibiotics. If clinical stability is achieved, the initial treatment can be considered effective. Clinical stability is defined by meeting all of the following five criteria:
- Body temperature ≤37.8°C
- Heart rate ≤100 beats/min
- Respiratory rate ≤24 breaths/min
- Systolic blood pressure ≥90 mmHg
- Oxygen saturation ≥90% (or arterial oxygen partial pressure ≥60 mmHg on room air).
For patients who achieve clinical stability and can tolerate oral medications, a step-down to oral therapy with the same or similar-spectrum antibiotics effective against the pathogen is recommended.
If symptoms do not improve in 72 hours, possible reasons include:
- Antibiotics do not cover the causative pathogen, or the pathogen is resistant to the drug.
- Infection with special pathogens, such as Mycobacterium tuberculosis, fungi, or viruses.
- Complications or host factors affecting treatment efficacy (e.g., immunosuppression).
- Misdiagnosis of a non-infectious disease as pneumonia.
- Drug fever.
A thorough analysis, necessary diagnostic tests, and appropriate management should be performed to address these issues.
Prevention
Preventive measures include regular physical exercise to improve overall fitness and reducing risk factors such as smoking and excessive alcohol consumption. Influenza vaccination is recommended for individuals over 65 years of age. Pneumococcal vaccination is recommended for individuals over 65 years or those under 65 with conditions such as cardiovascular disease, pulmonary disease, diabetes, alcoholism, liver cirrhosis, or immunosuppression.