Hypertension is a cardiovascular syndrome characterized by elevated systemic arterial pressure as its primary clinical manifestation. It can be classified into primary hypertension and secondary hypertension. Primary hypertension, also known as essential hypertension, is one of the most significant risk factors for cardiovascular and cerebrovascular diseases. It often coexists with other cardiovascular risk factors and can damage the structure and function of vital organs such as the heart, brain, and kidneys, ultimately leading to organ failure.
Blood Pressure Classification and Definition
Blood pressure in the population follows a continuous normal distribution. The diagnostic criteria for hypertension are defined based on clinical and epidemiological data. The current classification and standards for blood pressure are shown in the following table.
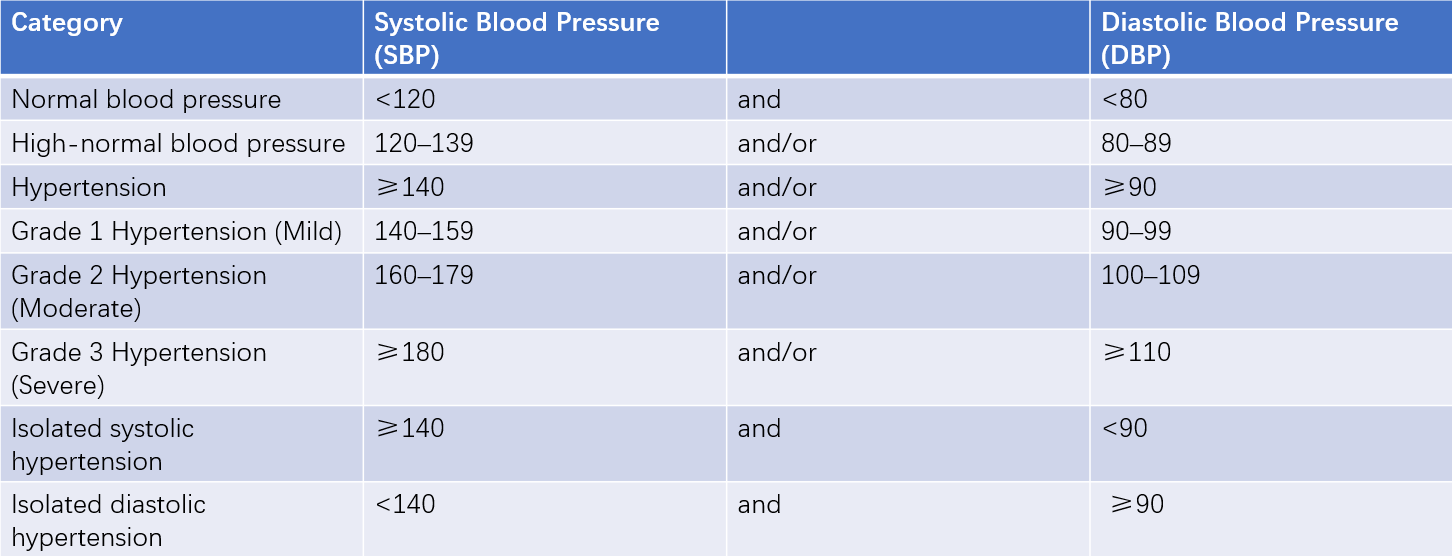
Table 1 Blood pressure classification and definition (mmHg)
Note: When systolic and diastolic blood pressures fall into different categories, the higher category is used for classification. These standards apply to adult males and females of any age.
Epidemiology
The prevalence and incidence of hypertension vary across countries, regions, and ethnic groups, with higher rates in industrialized nations compared to developing countries. The prevalence, incidence, and blood pressure levels increase with age. Hypertension is more common in older adults, particularly isolated systolic hypertension.
Etiology and Pathogenesis
Primary hypertension results from the interaction of genetic and environmental factors. It is not a homogeneous disease, and the causes and mechanisms of hypertension vary among individuals. The disease course of hypertension is typically long and progresses slowly. The initiating, sustaining, and accelerating mechanisms differ at various stages of the disease, with interactions between these mechanisms. Therefore, hypertension is a disease characterized by multiple factors, pathways, stages, mechanisms, and significant individual variability.
Factors Associated with Hypertension Development
Genetic Factors
Hypertension exhibits clear familial clustering. If both parents have hypertension, the likelihood of their offspring developing the condition is as high as 46%. Approximately 60% of hypertensive patients have a family history of hypertension. The genetic inheritance of hypertension may involve both dominant inheritance of major genes and polygenic inheritance. Genetically, not only is the incidence of hypertension heritable, but blood pressure levels, the occurrence of complications, and other related factors such as obesity also show genetic predispositions. In recent years, over 1,000 single nucleotide polymorphisms (SNPs) related to the genetic background of hypertension have been identified.
Environmental Factors
Blood pressure levels and hypertension prevalence in different populations are significantly positively correlated with average sodium intake. Excessive salt intake primarily raises blood pressure in salt-sensitive individuals. Potassium intake is negatively correlated with blood pressure. High protein intake is considered a hypertensive factor. Diets high in saturated fatty acids or with a high saturated fatty acid-to-polyunsaturated fatty acid ratio are also associated with elevated blood pressure. Alcohol consumption is linearly related to blood pressure levels, particularly systolic blood pressure.
The prevalence of hypertension is higher in mental laborers than in manual laborers. Individuals with high-stress occupations are more likely to develop hypertension. Prolonged exposure to noisy environments and hearing sensitivity loss are also associated with higher rates of hypertension.
Smoking increases the release of norepinephrine at sympathetic nerve endings, raising blood pressure. It also induces oxidative stress, impairing nitric oxide (NO)-mediated vasodilation, which contributes to elevated blood pressure.
Other Factors
Weight gain is a significant risk factor for elevated blood pressure. The type of obesity is closely associated with hypertension, with abdominal obesity being more strongly linked to hypertension.
Females taking oral contraceptives have an increased risk of developing hypertension, with the incidence and severity related to the duration of use. Hypertension caused by oral contraceptives is usually mild and reversible, with blood pressure often returning to normal 3-6 months after discontinuation. Other medications, such as ephedrine, corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and licorice, may also raise blood pressure.
Sleep apnea hypopnea syndrome (SAHS) is characterized by recurrent episodes of apnea or hypopnea during sleep and can be classified as either central or obstructive. Approximately 50% of SAHS patients have hypertension, with the degree of blood pressure elevation correlating with the duration and severity of SAHS.
Exposure to pollutants such as PM2.5, PM10, SO2, and O3 is associated with an increased risk of hypertension and higher cardiovascular mortality rates.
Pathogenesis
Neuronal Mechanisms
Various factors can alter the function of subcortical neural centers in the brain, leading to abnormal concentrations and activity of neurotransmitters, including norepinephrine, epinephrine, dopamine, neuropeptide Y, serotonin, vasopressin, enkephalins, brain natriuretic peptides, and the central renin-angiotensin system. These changes ultimately result in hyperactivity of the sympathetic nervous system.
Increased sympathetic excitability affects the heart, causing faster heart rate, enhanced myocardial contractility, and increased cardiac output. When acting on vascular alpha receptors, it induces small artery constriction, raises peripheral vascular resistance, and elevates blood pressure. Enhanced renal sympathetic nerve activity promotes sodium and water reabsorption via alpha-1 receptors in the proximal tubules, reduces renal blood flow by causing renal vasoconstriction, and activates beta-1 receptors to stimulate renin release, leading to the production of angiotensin II (AT II). AT II causes vasoconstriction, increased norepinephrine release, and enhanced sodium reabsorption. It also acts on the rostral ventrolateral medulla to activate renal sympathetic nerves, creating a positive feedback loop. These factors collectively increase cardiac output and peripheral resistance, thereby raising blood pressure.
Renal Mechanisms
Various factors can cause renal sodium and water retention, increasing cardiac output. Through systemic autoregulation, this leads to elevated peripheral vascular resistance and blood pressure. The pressure-natriuresis mechanism is then activated to excrete the retained sodium and water. Additionally, increased secretion of natriuretic hormones, such as endogenous digitalis-like substances, may promote sodium and water excretion while simultaneously increasing peripheral vascular resistance, thereby elevating blood pressure.
This theory suggests that elevated blood pressure may serve as a compensatory mechanism to maintain water and sodium balance in the body. Modern high-salt diets, combined with genetic or acquired impairments in renal sodium excretion, underlie the fundamental pathophysiological abnormalities in many hypertensive patients.
Several factors contribute to renal sodium and water retention, including increased renal vascular resistance due to sympathetic hyperactivity, microstructural changes in glomeruli, reduced secretion of renal natriuretic hormones (e.g., prostaglandins, kallikrein, medullipin), abnormal secretion of extrarenal natriuretic hormones (e.g., endogenous digitalis-like substances, thrombomodulin), and increased release of sodium-retaining hormones (e.g., 18-hydroxydeoxycorticosterone, aldosterone). Additionally, low birth weight can lead to hypertension through renal mechanisms.
Hormonal Mechanisms
The activation of the renin-angiotensin-aldosterone system (RAAS) plays a key role in hypertension. The classical RAAS pathway involves the secretion of renin by juxtaglomerular cells in the afferent arterioles of the kidney. Renin activates angiotensinogen (AGT) produced by the liver, forming angiotensin I (Ang I), which is then converted to angiotensin II (Ang II) by angiotensin-converting enzyme (ACE) in the lungs.
Ang II is the primary effector of RAAS and acts on angiotensin II type 1 receptors (AT1 receptors) to cause small artery smooth muscle contraction, stimulate aldosterone secretion from the adrenal cortex, and enhance norepinephrine release via positive feedback on presynaptic sympathetic nerve terminals. These effects collectively increase blood pressure.
Recent studies have identified RAAS components in various tissues, including the vascular wall, heart, central nervous system, kidneys, and adrenal glands. The tissue RAAS may play a more significant role in hypertension and target organ damage than the systemic RAAS. Furthermore, Ang I and Ang II can generate Angiotensin-(1-7) [Ang-(1-7)] through multiple pathways. Ang-(1-7) exerts vasodilatory and anti-proliferative effects on vascular smooth muscle cells by acting on G-protein-coupled MAS receptors.
Vascular Mechanisms
Structural and functional changes in large and small arteries, known as vascular remodeling, play a crucial role in the development of hypertension. Endothelial cells lining the vascular wall can generate, activate, and release various vasoactive substances, such as nitric oxide (NO), prostacyclin (PGI2), endothelin-1 (ET-1), and endothelium-derived contracting factors (EDCF), which regulate cardiovascular function.
Aging and various cardiovascular risk factors, such as dyslipidemia, hyperglycemia, smoking, and hyperhomocysteinemia, impair endothelial cell function. This leads to increased production of reactive oxygen species, enhanced NO inactivation, vascular inflammation, and oxidative stress, which affect the elasticity and structure of arteries.
Reduced arterial elasticity accelerates pulse wave velocity, causing the reflective wave to return to the central arteries during systole instead of diastole. This results in a delayed systolic pressure peak, elevated systolic blood pressure, decreased diastolic blood pressure, and widened pulse pressure. Structural (e.g., reduced number of small arteries or increased wall-to-lumen ratio) and functional (e.g., reduced elasticity and increased resistance) changes in resistance arterioles alter the position of peripheral pressure reflection points or the intensity of reflected waves, further contributing to pulse pressure widening.
Insulin Resistance
Insulin resistance (IR) refers to a condition in which higher-than-normal blood insulin levels are required to maintain normal glucose tolerance, indicating reduced ability of body tissues to utilize insulin for glucose metabolism. Approximately 50% of patients with primary hypertension exhibit varying degrees of IR, which is most pronounced in individuals with the metabolic syndrome, characterized by obesity, elevated triglycerides, hypertension, and impaired glucose tolerance.
IR is now recognized as a shared pathophysiological basis for type 2 diabetes and hypertension. IR-induced hyperinsulinemia enhances renal sodium and water reabsorption, increases sympathetic nervous system activity, and reduces arterial elasticity, all of which contribute to elevated blood pressure.
To some extent, the sympathetic hyperactivity caused by IR increases thermogenesis as a compensatory mechanism against obesity, but this comes at the cost of elevated blood pressure and dyslipidemia.
Other Factors
Inflammation, immune responses, and various other factors also contribute to the development of hypertension and target organ damage.
Pathophysiology and Pathology
From a hemodynamic perspective, mean arterial blood pressure (MBP) is determined by the equation: MBP = cardiac output (CO) × total peripheral resistance (PR).
As individuals age, different hemodynamic characteristics are observed.
In young hypertensive individuals, the primary hemodynamic change is an increase in cardiac output and aortic stiffness, reflecting excessive activation of the sympathetic nervous system. This is more commonly seen in males.
Middle-aged patients often exhibit increased diastolic blood pressure, with or without elevated systolic blood pressure. Isolated diastolic hypertension is common in middle-aged males and is often associated with weight gain. The main hemodynamic feature is increased peripheral vascular resistance, while cardiac output remains normal.
Isolated systolic hypertension is the most common type in older individuals. Epidemiological studies show that systolic blood pressure increases with age, while diastolic blood pressure peaks around age 55 and then gradually declines. The widening of pulse pressure suggests central arterial stiffening and accelerated return of peripheral arterial wave reflections, leading to increased systolic blood pressure. Isolated systolic hypertension is more common in older individuals and females.
The heart and blood vessels are the primary target organs affected by hypertension. In the early stages, there may be no significant pathological changes. Long-term hypertension, however, leads to cardiac changes such as left ventricular hypertrophy and dilation. Systemic small artery damage is characterized by an increased wall-to-lumen ratio and narrowed luminal diameter, resulting in ischemia in vital organs such as the heart, brain, and kidneys. Chronic hypertension, along with associated risk factors, promotes the development of atherosclerosis. Endothelial dysfunction is the earliest and most critical vascular damage caused by hypertension.
Heart
Chronic pressure overload, along with the effects of catecholamines and angiotensin II (AT II), can stimulate myocardial hypertrophy and interstitial fibrosis, resulting in left ventricular hypertrophy and dilation, a condition known as hypertensive heart disease. Left ventricular hypertrophy reduces coronary blood flow reserve, particularly during increased oxygen demand, leading to subendocardial ischemia. Hypertensive heart disease is often accompanied by coronary atherosclerosis and coronary microvascular dysfunction.
Brain
Chronic hypertension causes ischemia and degeneration of cerebral blood vessels, leading to the formation of microaneurysms, which can rupture and cause cerebral hemorrhage. Hypertension promotes cerebral atherosclerosis, and plaque rupture can result in cerebral thrombosis. Occlusive lesions in small cerebral arteries can cause lacunar infarctions. Hypertensive cerebrovascular lesions are particularly prone to occur in the lenticulostriate arteries of the middle cerebral artery, the paramedian arteries of the basilar artery, and the dentate nucleus arteries of the cerebellum. These vessels originate directly from high-pressure large arteries, are slender, and penetrate vertically, making them susceptible to microaneurysms or occlusive lesions. Thus, strokes commonly affect regions such as the putamen, thalamus, caudate nucleus, and internal capsule.
Kidneys
Chronic hypertension leads to increased pressure in the glomerular capsule, resulting in glomerular fibrosis, atrophy, and renal artery sclerosis. These changes cause renal parenchymal ischemia and a progressive reduction in nephron numbers. Chronic kidney failure is a severe consequence of long-term hypertension, particularly in patients with coexisting diabetes. In malignant hypertension, proliferative endarteritis and fibrinoid necrosis occur in the afferent arterioles and interlobular arteries, potentially leading to rapid-onset renal failure.
Retina
Fundus examination provides insight into the severity of hypertension. The Keith-Wagener classification is commonly used to grade hypertensive retinopathy:
- Grade I: Narrowing of retinal arterioles with enhanced light reflex
- Grade II: Retinal arteriolar narrowing with arteriovenous crossing changes (arteriovenous nicking)
- Grade III: Retinal hemorrhages and cotton-wool spots in addition to the above changes
- Grade IV: Optic disc edema in addition to the above changes
Clinical Manifestations
Symptoms
Most hypertensive patients have a slow onset and lack specific clinical manifestations, leading to delayed diagnosis. Hypertension is often discovered during blood pressure measurement or after complications involving the heart, brain, or kidneys have occurred. Common symptoms include dizziness, headache, neck stiffness, fatigue, and palpitations. Severe symptoms may include blurred vision and epistaxis. Classic hypertensive headaches typically resolve after blood pressure is lowered.
Hypertensive patients may also experience headaches unrelated to blood pressure levels, such as tension headaches, migraines, and those caused by glaucoma. Sudden severe dizziness or vertigo may indicate cerebrovascular disease, excessive blood pressure reduction, or orthostatic hypotension. Symptoms related to target organ damage, such as chest tightness, shortness of breath, angina, and polyuria, may also be present. Additionally, some symptoms may result from adverse effects of antihypertensive medications.
Signs
Physical signs of hypertension are generally minimal. Examination should focus on peripheral vascular pulsations, vascular bruits, and cardiac murmurs. Particular attention should be paid to vascular bruits in the neck, bilateral costovertebral angles, upper abdomen near the umbilicus, and lumbar costovertebral regions.
Cardiac auscultation may reveal accentuated second heart sounds in the aortic valve area, systolic murmurs, or early systolic ejection clicks.
Certain signs may suggest secondary hypertension. For example, lumbar masses suggest polycystic kidney disease or pheochromocytoma; delayed or absent femoral artery pulsations with lower blood pressure in the legs compared to the arms suggest coarctation of the aorta; centripetal obesity, striae, and hirsutism suggest Cushing's syndrome.
Complications
Common complications include:
- Cerebrovascular diseases, including cerebral hemorrhage, cerebral thrombosis, lacunar infarctions, and transient ischemic attacks
- Heart failure and coronary artery disease
- Chronic kidney failure
- Aortic dissection
Laboratory Tests
Basic tests include:
- Blood biochemistry: sodium, potassium, fasting blood glucose, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), uric acid, and creatinine
- Complete blood count (CBC): hemoglobin and hematocrit
- Urinalysis: protein, glucose, and microscopic examination of urinary sediment
- Electrocardiogram (ECG)
Recommended tests include:
- 24-hour ambulatory blood pressure monitoring (ABPM)
- Echocardiography
- Carotid ultrasound
- Postprandial 2-hour blood glucose
- Homocysteine levels
- Quantitative measurement of urinary albumin and urinary protein
- Fundus examination
- Chest x-ray
- Pulse wave velocity (PWV)
- Ankle-brachial index (ABI)
ABPM involves the automatic measurement of blood pressure at regular intervals (every 15-30 minutes) over 24 hours or longer. In healthy individuals, blood pressure follows a distinct circadian rhythm, characterized by two peaks (6-10 AM and 4-8 PM) and a trough at night, during which blood pressure significantly decreases.
ABPM can be used to:
- Diagnose white-coat hypertension
- Identify masked hypertension
- Detect resistant hypertension
- Assess the degree of blood pressure elevation, short-term variability, circadian rhythm, and treatment efficacy
For patients suspected of secondary hypertension, the following tests can be selected as needed:
- Plasma renin activity or concentration
- Blood and urine aldosterone levels
- Blood and urine cortisol levels
- Blood and urine catecholamines and their metabolites
- Arteriography
- Renal and adrenal ultrasound
- Adrenal CT or MRI
- Sleep apnea monitoring
For hypertensive patients with complications, additional evaluations of the heart, brain, kidneys, and other organs should be conducted as appropriate.
Diagnosis and Differential Diagnosis
The diagnosis of hypertension is based on blood pressure measurements taken in a clinical setting using a validated mercury sphygmomanometer or electronic blood pressure monitor. Blood pressure should be measured in the brachial artery of the upper arm in a seated, resting position. Hypertension can be diagnosed if the systolic blood pressure (SBP) is consistently ≥140 mmHg and/or diastolic blood pressure (DBP) is consistently ≥90 mmHg on at least three separate occasions on different days.
Patients with a history of hypertension who are currently on antihypertensive medication are also diagnosed with hypertension, even if their blood pressure is within the normal range.
Hypertension can also be diagnosed based on the following criteria:
- Home blood pressure monitoring: SBP ≥135 mmHg and/or DBP ≥85 mmHg
- 24-hour ABPM
- Mean SBP ≥130 mmHg and/or mean DBP ≥80 mmHg
- Daytime mean SBP ≥135 mmHg and/or mean DBP ≥85 mmHg
- Nighttime mean SBP ≥120 mmHg and/or mean DBP ≥70 mmHg
Generally, the difference in blood pressure between the left and right arms is <10-20 mmHg. If a significant discrepancy exists, obstructive lesions in the subclavian artery or its distal branches should be considered. For patients suspected of orthostatic hypotension, blood pressure should be measured in both the supine and standing positions.
A diagnosis of hypertension should not be based solely on one or two office blood pressure readings. Instead, blood pressure should be monitored over time, with follow-up assessments to observe changes and establish an overall trend. For accurate diagnosis and long-term management, it is essential to complement office blood pressure measurements with home blood pressure monitoring and ABPM to comprehensively evaluate the patient’s blood pressure profile and achieve better control.
In line with the World Health Organization's (WHO) initiative to reduce mercury pollution, electronic blood pressure monitors are expected to become the primary tools for blood pressure measurement in the future. Advances in technology will further improve the accuracy and convenience of blood pressure measurement. Remote blood pressure monitoring and non-invasive beat-to-beat blood pressure measurement have already been introduced in clinical practice.
Once hypertension is diagnosed, it is crucial to differentiate between primary (essential) and secondary hypertension.
Risk Assessment and Prognosis
The prognosis of hypertensive patients depends not only on blood pressure levels but also on the presence of other cardiovascular risk factors and the extent of target organ damage. To guide treatment and assess prognosis, cardiovascular risk stratification should be performed, categorizing patients into low, moderate, high, and very high-risk groups.
Stratification is based on the level of blood pressure elevation (grades 1, 2, or 3), the presence of other cardiovascular risk factors, diabetes, chronic kidney disease (CKD), target organ damage, and complications.
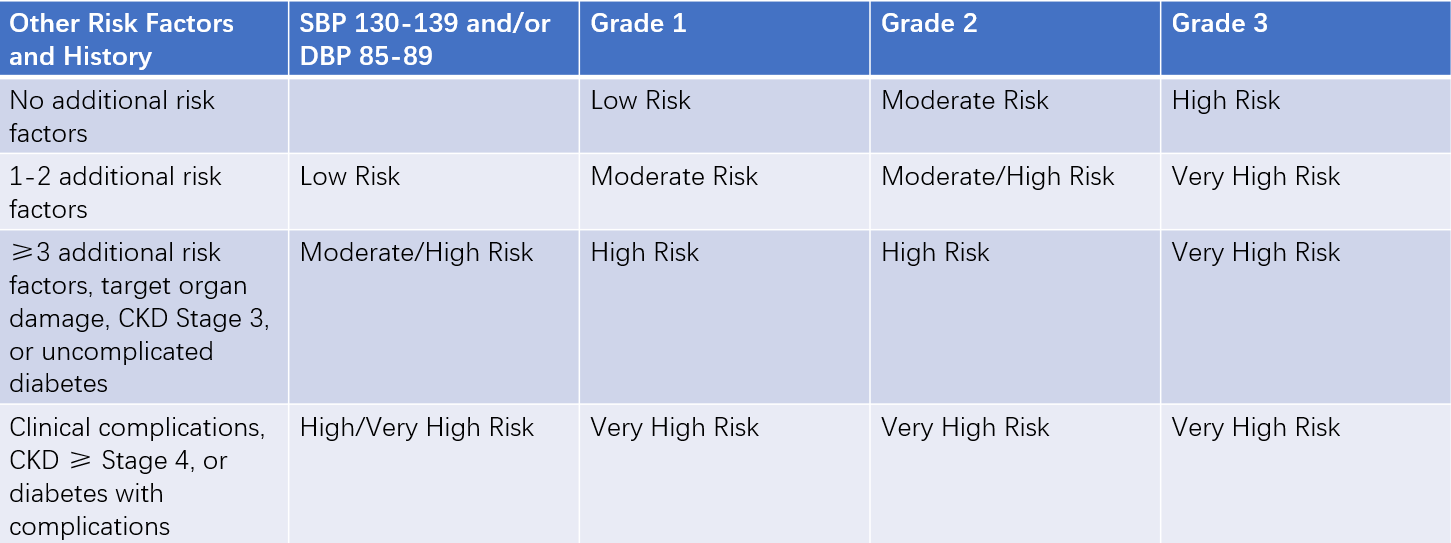
Table 2 Cardiovascular risk stratification for hypertensive patients
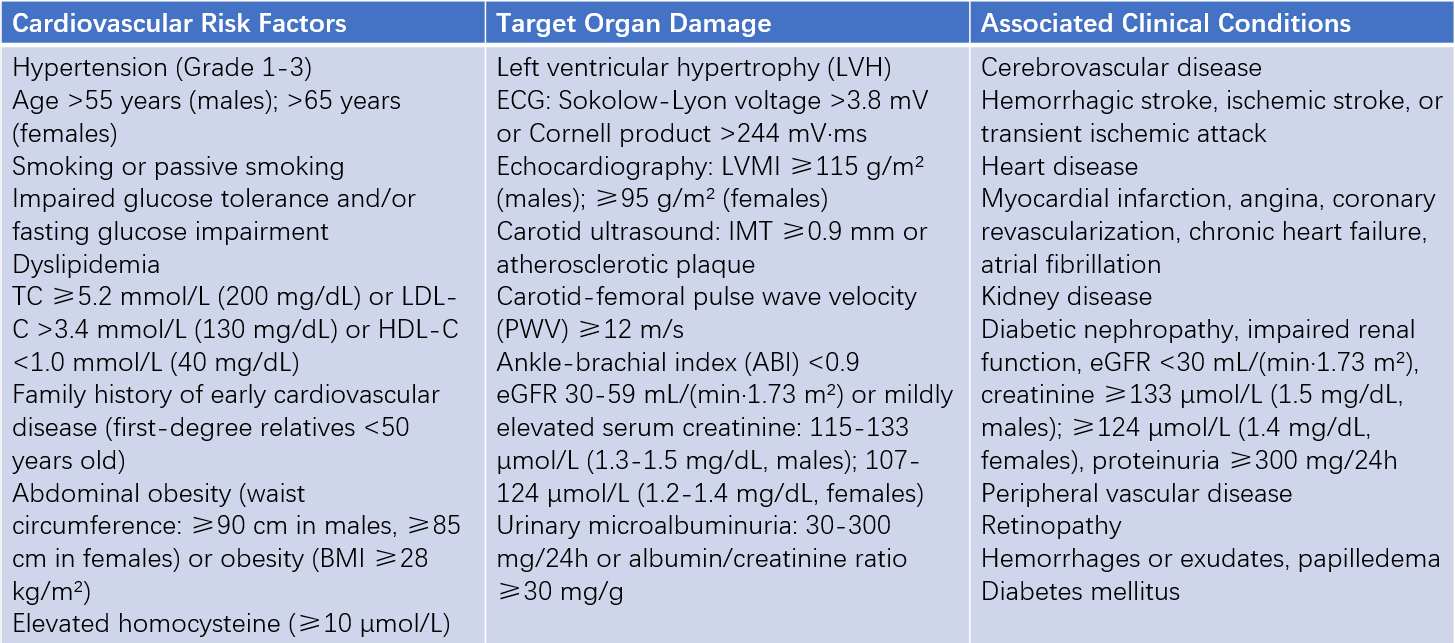
Table 3 Key factors affecting cardiovascular prognosis in hypertensive patients
Abbreviations: LVMI, Left Ventricular Mass Index; IMT, Intima-Media Thickness; ABI, Ankle-Brachial Index; PWV, Pulse Wave Velocity.
Treatment
Objectives and Principles
Currently, there is no cure for primary hypertension. Clinical evidence shows that a reduction in systolic blood pressure (SBP) by 10-20 mmHg or diastolic blood pressure (DBP) by 5-6 mmHg over 3-5 years can reduce the incidence of stroke, coronary heart disease, and cardiovascular mortality by 38%, 16%, and 20%, respectively, while heart failure can be reduced by over 50%. High-risk patients benefit even more significantly. The ultimate goal of antihypertensive treatment is to reduce the incidence and mortality of cardiovascular and cerebrovascular diseases in hypertensive patients.
Lifestyle interventions are recommended for all hypertensive patients:
- Weight reduction: maintaining a body mass index (BMI) below 24 kg/m2
- Reducing sodium intake: limiting daily salt consumption to no more than 6 g
- Increasing potassium intake: consuming fresh vegetables and fruits daily
- Reducing fat intake: limiting the consumption of cooking oil and avoiding fatty meats and animal organs
- Quitting smoking and limiting alcohol consumption
- Increasing physical activity
- Reducing psychological stress and maintaining emotional balance
- Supplementing folic acid if necessary
Patients whose blood pressure remains above 140/90 mmHg and/or above target levels despite lifestyle modifications should receive drug therapy:
- High-risk and very high-risk patients: Initiate antihypertensive drug treatment promptly
- Moderate-risk patients: Observe for several weeks, assess target organ damage, and improve lifestyle. If blood pressure remains uncontrolled, initiate drug therapy
- Low-risk patients: Observe for 1-3 months with close follow-up, preferably with out-of-office blood pressure monitoring, and assess target organ damage. If blood pressure remains uncontrolled despite lifestyle modifications, start drug therapy
The general target for blood pressure control is <140/90 mmHg. For patients with diabetes, chronic kidney disease (CKD), heart failure, or stable coronary artery disease, the target is <130/80 mmHg.
For older patients with isolated systolic hypertension, the systolic blood pressure should be controlled to below 150 mmHg, and if tolerated, further reduced to below 140 mmHg. Blood pressure should be lowered to the target levels as early as possible, but not too rapidly.
For most hypertensive patients, blood pressure should be gradually reduced to target levels within 4-12 weeks, depending on their condition. Younger patients or those with a shorter disease duration can achieve target levels more quickly. For older patients, those with a longer disease duration, or those with target organ damage or complications, the rate of blood pressure reduction should be moderate and slower.
There is a synergistic effect among various cardiovascular risk factors, and most hypertensive patients have additional cardiovascular risk factors. Even after achieving appropriate blood pressure control, other risk factors may still significantly affect prognosis. Therefore, antihypertensive treatment should also address other cardiovascular risk factors.
In addition to effectively lowering blood pressure, treatment strategies should aim to control multiple risk factors, including blood glucose, blood lipids, uric acid, and homocysteine levels.
Antihypertensive Drug Therapy
The use of antihypertensive drugs should follow four key principles: individualization, initial dose selection, preference for long-acting formulations, and combination therapy.
Individualization
Treatment should be tailored to the patient’s specific conditions, blood pressure level, risk stratification, comorbidities, drug efficacy, and tolerability. Economic considerations and patient preferences should also be taken into account. The choice of antihypertensive drugs may involve either monotherapy or combination therapy as the initial approach.
Initial Dose Selection
For most patients, standard doses are typically used. For older patients, treatment should generally start with lower effective doses. The dose can be gradually increased to the full therapeutic level as needed. In some cases, initial combination therapy can be considered.
Preference for Long-acting Formulations
Long-acting drugs that require once-daily dosing and provide 24-hour blood pressure control are preferred. These drugs effectively control nighttime and morning surge blood pressure, better prevent cardiovascular and cerebrovascular complications, and improve treatment adherence.
Combination Therapy
Combination therapy can enhance blood pressure control without significantly increasing adverse effects. If low-dose monotherapy is insufficient, two or more antihypertensive drugs can be combined. For patients with blood pressure ≥160/100 mmHg or 20/10 mmHg above the target level, or those at high or very high risk, initial combination therapy—either free-drug combinations or fixed-dose combination (FDC) formulations—can be used. FDCs are particularly beneficial for improving blood pressure control rates.
The commonly used classes of antihypertensive drugs include:
- Diuretics
- Beta-blockers
- Calcium channel blockers (CCBs)
- Angiotensin-converting enzyme inhibitors (ACEIs)
- Angiotensin II receptor blockers (ARBs)
- Angiotensin receptor-neprilysin inhibitors (ARNIs)
Diuretics
Diuretics are classified into three major types: thiazide diuretics, loop diuretics, and potassium-sparing diuretics.
Common thiazide diuretics include hydrochlorothiazide. These drugs lower blood pressure primarily by promoting sodium excretion, reducing extracellular fluid volume, and decreasing peripheral vascular resistance. The antihypertensive effect is gradual and sustained over a relatively long duration. They are particularly effective in patients with isolated systolic hypertension, salt-sensitive hypertension, obesity, diabetes, heart failure, or older patients with hypertension.
Thiazide diuretics can enhance the efficacy of other antihypertensive drugs. However, their main adverse effects include hypokalemia and disturbances in lipid, glucose, and uric acid metabolism, which are more likely at higher doses. Therefore, low doses are recommended. Other side effects include fatigue and increased urination. They are contraindicated in patients with gout.
Potassium-sparing diuretics can cause hyperkalemia and should be used cautiously with ACEIs or ARBs and in patients with renal insufficiency.
Loop diuretics are primarily used in hypertensive patients with renal insufficiency.
Beta-Blockers
Beta-blockers are categorized into three types: selective (β1), non-selective (β1 and β2), and those with combined alpha-blocking properties.
These drugs lower blood pressure by inhibiting the central and peripheral renin-angiotensin-aldosterone system (RAAS), reducing myocardial contractility, and slowing heart rate. They have a strong and rapid antihypertensive effect, with varying durations of action depending on the specific beta-blocker. They are suitable for middle-aged or younger patients with a faster heart rate, or those with comorbid conditions such as angina pectoris and chronic heart failure. Their efficacy in older hypertensive patients is relatively lower.
Clinically, selective β1 blockers or those with combined alpha-blocking properties are preferred to achieve effective heart rate reduction at higher doses. Beta-blockers not only lower resting blood pressure but also prevent sharp increases in blood pressure during physical stress or exercise.
Although diabetes is not an absolute contraindication for beta-blockers, they may increase insulin resistance and mask or prolong hypoglycemic episodes, requiring careful monitoring.
Abrupt discontinuation of beta-blockers at high doses may lead to withdrawal syndrome.
Common side effects include bradycardia, fatigue, and cold extremities.
Beta-blockers should not be used in patients with acute heart failure, sick sinus syndrome, or severe atrioventricular conduction block. They also increase airway resistance and should be avoided in patients with respiratory conditions such as asthma.
Calcium Channel Blockers
CCBs are divided into dihydropyridines (e.g., nifedipine) and non-dihydropyridines (e.g., verapamil and diltiazem). Based on the duration of action, CCBs can also be categorized into short-acting and long-acting formulations.
Long-acting CCBs include:
- Long half-life drugs: e.g., amlodipine and lercanidipine
- Lipid-soluble membrane-controlled drugs: e.g., lacidipine and lercanidipine
- Sustained-release or controlled-release formulations: e.g., felodipine sustained-release tablets and nifedipine controlled-release tablets
The antihypertensive effect of CCBs is primarily achieved by blocking voltage-dependent L-type calcium channels, reducing extracellular calcium ion influx into vascular smooth muscle cells. This decreases excitation-contraction coupling and reduces the contraction response of resistance vessels. CCBs also mitigate the vasoconstrictive effects of angiotensin II (ATII) and α1 adrenergic receptors, as well as reduce renal tubular sodium reabsorption.
Advantages of CCBs include:
- Rapid onset of action with strong and consistent antihypertensive effects
- Minimal individual variability in response
- Effective in combination with other antihypertensive drugs, significantly enhancing blood pressure control
- No significant impact on lipid or glucose metabolism, making them well-tolerated with good adherence
Compared to other antihypertensive drugs, CCBs offer additional benefits:
- Effective in older patients
- Efficacy not compromised by high sodium intake or the use of nonsteroidal anti-inflammatory drugs (NSAIDs)
- Effective in patients with alcohol dependence
- Indications for patients with diabetes, coronary artery disease, or peripheral vascular disease
- Long-term use with anti-atherosclerotic effects
Reflex sympathetic activation at the start of treatment may cause increased heart rate, facial flushing, headache, and lower extremity edema, particularly with short-acting formulations. Non-dihydropyridine CCBs inhibit myocardial contraction and conduction and are not recommended for patients with reduced ejection fraction heart failure, sinoatrial dysfunction, or severe cardiac conduction block.
Angiotensin-converting Enzyme Inhibitors
The antihypertensive effect of ACEIs is primarily achieved by inhibiting circulating and tissue ACE, reducing the production of angiotensin II (ATII), and simultaneously inhibiting kininase, which decreases the degradation of bradykinin.
Onset of action is gradual, reaching maximum effect in 3-4 weeks. Sodium restriction or combination with diuretics can accelerate onset and enhance efficacy.
ACEIs improve insulin resistance and reduce proteinuria, making them effective for hypertensive patients with obesity, diabetes, or target organ damage (e.g., heart or kidneys). They are particularly suitable for patients with conditions such as heart failure, myocardial infarction, atrial fibrillation, proteinuria, impaired glucose tolerance, and diabetic nephropathy.
The most common side effects are dry cough and angioedema. Dry cough occurs in 10-20% of patients and is likely related to increased bradykinin levels; it typically resolves after discontinuation.
ACEIs should not be used in pregnant women, patients with bilateral renal artery stenosis, hyperkalemia, or a history of angioedema.
Angiotensin II Receptor Blockers
The antihypertensive effect of ARBs is achieved by blocking tissue AT1 receptors, effectively preventing the vasoconstrictive, sodium-retentive, and remodeling effects of angiotensin II.
Blocking AT1 receptors leads to a feedback increase in angiotensin II, which can activate AT2 receptors, further counteracting the biological effects of AT1 receptors.
Onset of action is gradual but sustained and stable. A low-salt diet or combination with diuretics can significantly enhance efficacy.
Most ARBs exhibit a dose-dependent antihypertensive effect with a wide therapeutic window.
ARBs are associated with fewer drug-specific adverse effects. Unlike ACEIs, ARBs generally do not cause dry cough, leading to better long-term adherence.
Indications and contraindications are similar to ACEIs.
Angiotensin Receptor-neprilysin Inhibitors
Sacubitril/valsartan is a novel crystalline compound composed of the neprilysin inhibitor sacubitril and valsartan in a 1:1 molar ratio.
Compared to combination formulations, the crystalline structure ensures a consistent composition, better storage stability, and significantly improved solubility and oral bioavailability.
Mechanism includes:
- Blocking the effects of angiotensin II on AT1 receptors
- Inhibiting the degradation of natriuretic peptides, enhancing their antihypertensive effects
Natriuretic peptides promote peripheral vasodilation, increase sodium excretion by the kidneys, and inhibit the renin-angiotensin-aldosterone system (RAAS), aldosterone, and vasopressin. They also suppress sympathetic nervous activity, contributing to blood pressure reduction.
Indications and contraindications are similar to ACEIs.
Other Antihypertensive Drugs
In addition to the six major classes of antihypertensive drugs, other agents have been used historically, including:
- Centrally acting antihypertensive drugs: e.g., reserpine, clonidine
- Vascular smooth muscle relaxants: e.g., hydralazine
- α1 receptor blockers: e.g., prazosin, terazosin, doxazosin
These drugs were widely used in the past but are now less commonly recommended due to their side effects. They may still be used in combination therapies or fixed-dose formulations.
Direct renin inhibitors, a newer class of antihypertensive drugs, can significantly lower blood pressure. However, their impact on cardiovascular outcomes requires further evaluation through large-scale clinical trials.
Antihypertensive Treatment Strategies
Most patients without complications can be treated with monotherapy or combination therapy using thiazide diuretics, beta-blockers, CCBs, ACEIs, ARBs, or ARNIs.
Comorbid cardiovascular risk factors, target organ damage, complications, antihypertensive efficacy, adverse effects, and drug costs may all affect the choice of antihypertensive drugs.
For patients with grade 2 hypertension, combination therapy can be initiated from the start.
Combination therapy helps achieve target blood pressure more quickly and reduces the likelihood of adverse effects.
Combination therapy should involve antihypertensive drugs with different mechanisms of action. The primary recommended combination therapy regimens include:
- ACEI/ARB + Dihydropyridine CCB
- ARB/ACEI + Thiazide diuretics
- Dihydropyridine CCB + Thiazide diuretics
- Dihydropyridine CCB + Beta-blockers
Secondary recommended combination therapy regimens include:
- Diuretics + Beta-blockers
- Alpha-receptor blockers + Beta-blockers
- Dihydropyridine CCB + Potassium-sparing diuretics
- Thiazide diuretics + Potassium-sparing diuretics
Combination therapy can also involve the use of single-pill combination (SPC) formulations, which typically consist of two or more antihypertensive drugs with different mechanisms of action. Compared to free combinations, SPCs are more convenient to use, improving treatment adherence and efficacy. Commonly used SPCs include:
- ACEI/ARB + Dihydropyridine CCB
- ARB/ACEI + Thiazide diuretics
- Dihydropyridine CCB + Beta-blockers
When three antihypertensive drugs are combined, the regimen should generally include a diuretic. With a rational treatment plan and good adherence, most patients can achieve their target blood pressure within 4-12 weeks.
Lifestyle interventions and pharmacological treatment are the fundamental approaches for managing hypertension. Renal denervation (RDN), a catheter-based interventional therapy, reduces sympathetic nerve activity. In recent years, multiple clinical studies have demonstrated the efficacy and safety of RDN in treating hypertension. It is particularly suitable for patients with poorly controlled or resistant hypertension and has been clinically adopted in several countries.
The benefits of antihypertensive treatment are primarily achieved through long-term blood pressure control. Therefore, hypertension patients, especially those at high or very high risk, require long-term antihypertensive therapy. Once an effective treatment plan has been established and blood pressure is controlled, treatment should be continued without arbitrary discontinuation or frequent changes to the regimen.
Given the long-term nature of antihypertensive therapy, patient adherence is critically important for achieving sustained blood pressure control and preventing complications.