Dilated cardiomyopathy (DCM) is a type of cardiomyopathy characterized by dilation of the left ventricle or both ventricles, accompanied by systolic dysfunction, which cannot be explained by abnormal loading conditions or myocardial ischemia. The incidence of DCM ranges from 0.13‰ to 0.84‰, and the prognosis is poor, with 5-year and 10-year survival rates of approximately 50% and 25%, respectively, after diagnosis.
Etiology and Pathogenesis
The exact cause of DCM remains unclear. DCM often exhibits a familial pattern, with inheritance most commonly being autosomal dominant, though autosomal recessive and X-linked inheritance patterns are also observed. Acquired factors such as viral infections and autoimmune mechanisms also contribute to disease development.
Genetic Mutations
Pathogenic gene mutations can be identified in approximately 50% of familial and 30% of sporadic DCM cases. Compared to hypertrophic cardiomyopathy (HCM), DCM involves a greater number of causative genes, with over 50 gene mutations reported. Among these, mutations in structural proteins of the myocardium, particularly truncating mutations in the titin gene (TTN), are the most significant. Mutations in sarcomeric, intercalated disc, desmosomal, and ion channel proteins have also been identified, with overlap seen in the pathogenic genes for HCM, arrhythmogenic right ventricular cardiomyopathy (ARVC), and cardiac ion channelopathies.
Viral Infections
Direct viral invasion and the resulting acute or chronic inflammation and immune responses are key mechanisms of myocardial injury and progression to DCM.
Cardiotoxic Substances
Alcohol abuse is a common cause of DCM. Chemotherapeutic agents and other cardiotoxic drugs, such as anthracycline-based anticancer drugs, lithium, and emetine, can also lead to DCM.
Immune Responses
Immune-mediated mechanisms are observed in conditions such as granulomatous myocarditis, hypersensitivity myocarditis, and connective tissue diseases. The immune responses associated with these conditions can directly or indirectly cause DCM.
Endocrine and Metabolic Disorders
Deficiencies in certain vitamins and trace elements, such as selenium, can result in DCM. Endocrine and metabolic diseases, including thyroid disorders and pheochromocytoma, are also common causes.
Other Causes
Neuromuscular diseases, such as Duchenne and Becker muscular dystrophies, are often associated with DCM.
Pathology and Pathophysiology
The ventricles appear dilated, with thinning of the ventricular walls, scar formation, and mural thrombi. However, there are no significant abnormalities in the valves or coronary arteries.
Non-specific findings include myocardial cell hypertrophy, degeneration, necrosis, fibrosis, and infiltration of inflammatory cells.
Weakened myocardial contractility triggers neurohormonal activation, leading to sodium and water retention, increased heart rate, and vasoconstriction to maintain circulation. However, excessive compensation results in further myocardial injury, eventually leading to decompensation due to a vicious cycle.
Clinical Manifestations
Symptoms
The main symptoms include exertional dyspnea and reduced exercise tolerance. In advanced stages, symptoms of left heart failure, such as paroxysmal nocturnal dyspnea and orthopnea, may develop, eventually progressing to symptoms of global heart failure. When arrhythmias are present, patients may experience palpitations, dizziness, or syncope. Persistent and refractory hypotension is often a sign of end-stage disease.
Signs
Physical examination may reveal cardiac enlargement, diminished heart sounds, and the presence of a third or fourth heart sound. When the heart rate is rapid, a gallop rhythm may be heard. A systolic murmur at the apex may sometimes be detected. In advanced stages, signs of left or global heart failure may be evident.
Auxiliary Examinations
Laboratory Tests
Testing for anti-myocardial antibodies can help determine whether DCM is associated with autoimmune mechanisms. Biomarkers such as B-type natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP) and cardiac troponins are crucial for assessing cardiac function, treatment efficacy, and prognosis.
Electrocardiogram (ECG)
ECG findings are non-specific and may include widened QRS complexes, poor R-wave progression, abnormal Q waves, ST-segment depression, T-wave inversion, bundle branch block, intraventricular conduction delays, and various tachyarrhythmias.
Chest X-ray
Findings may include cardiomegaly, pulmonary congestion, pulmonary edema, and pleural effusion.
Transthoracic Echocardiography (TTE)
Transthoracic echocardiography is the first-choice modality for evaluating DCM. Key findings include left ventricular dilation, reduced wall motion, and decreased left ventricular ejection fraction (LVEF). Diffuse hypokinesis of the ventricular wall is a relatively characteristic feature of DCM, distinguishing it from the segmental hypokinesis observed in ischemic cardiomyopathy.
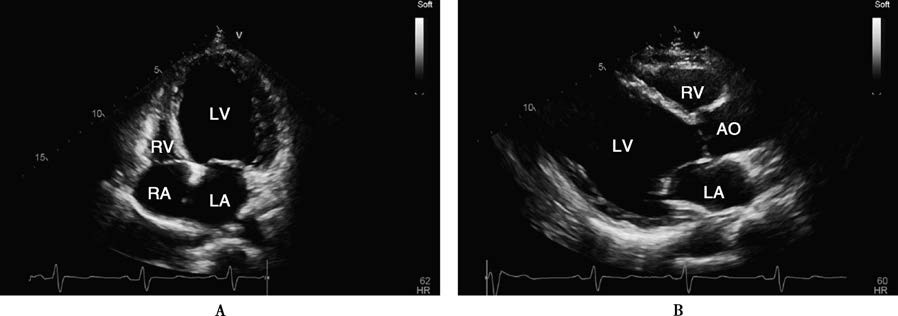
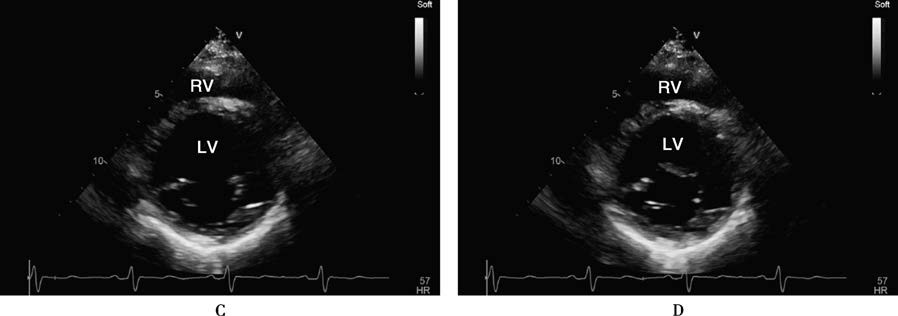
Figure 1 Echocardiographic findings in a patient with dilated cardiomyopathy
A 32-year-old male patient with dilated cardiomyopathy. The four-chamber view and left ventricular long-axis view show significant left ventricular cavity dilation at end-systole (A, B). The short-axis view at the mitral valve level demonstrates minimal changes in chamber size between end-systole (C) and end-diastole (D). Dynamic observation reveals globally severe hypokinesis of all ventricular wall segments, with a left ventricular ejection fraction (LVEF) of approximately 10%.
AO: Aorta; LA: Left atrium; LV: Left ventricle; RA: Right atrium; RV: Right ventricle.
Cardiac Magnetic Resonance (CMR)
CMR has high value for diagnosis, differential diagnosis, and prognosis assessment, though it is time-consuming and costly, making it unsuitable as a first-line test. CMR imaging with different sequences and contrast enhancement provides diagnostic information similar to TTE while offering superior tissue characterization. Findings such as late gadolinium enhancement (LGE) can predict all-cause mortality, heart failure hospitalization, and sudden cardiac death (SCD).
Genetic Testing
Over 50 genes have been associated with DCM, with the titin gene being the most significant in encoding cytoskeletal proteins. Genetic screening of family members aids in diagnosing hereditary DCM.
Diagnosis and Differential Diagnosis
Diagnostic Criteria
DCM can be suspected when ventricular dilation is accompanied by reduced LVEF, after excluding acquired causes.
Differential Diagnosis
Other secondary causes of cardiac enlargement and systolic dysfunction should be excluded. A combination of history, physical examination, TTE, CMR, coronary angiography, and, if necessary, endomyocardial biopsy (EMB) and genetic testing can aid in differentiation.
Notably, CMR provides valuable myocardial tissue characterization for differential diagnosis. Myocardial edema on CMR suggests myocarditis. Patterns of LGE distribution and severity can help confirm or exclude specific causes:
- Subendocardial or transmural LGE indicates myocardial infarction.
- Subepicardial LGE suggests myocarditis.
- Patchy LGE is seen in sarcoidosis.
- Extensive lateral wall LGE is characteristic of muscular dystrophies.
- Mid-septal LGE is associated with LMNA mutations.
- Annular LGE is seen in truncating mutations of DSP and FLNC genes.
Treatment
Modern treatment strategies for DCM include pharmacological therapy, device-based interventions, and heart transplantation. The goals are to mitigate myocardial damage caused by underlying etiologies, prevent ventricular remodeling and neurohormonal activation, address triggers of heart failure, control arrhythmias, prevent sudden death, manage complications, improve quality of life, and prolong survival.
Underlying causes should be treated, such as controlling infections, limiting or abstaining from alcohol, managing endocrine or autoimmune diseases, correcting nutritional deficiencies, and addressing anemia, volume overload, or electrolyte imbalances.
The new quadruple therapy includes renin-angiotensin system (RAS) inhibitors (ACEIs, ARBs, ARNI), beta-blockers, SGLT2 inhibitors, and mineralocorticoid receptor antagonists (MRAs). This combination has been shown to reduce mortality and improve prognosis. In the absence of contraindications, patients should start treatment as early as possible, beginning with low doses and titrating to target or maximum tolerated doses.
ACEIs/ARBs
All heart failure (HF) patients with LVEF < 40% should receive an ACEI or ARB unless contraindicated.
ARNI
For patients with HFrEF who remain symptomatic despite optimal treatment with ACEI/ARB, beta-blockers, and MRAs, replacing ACEI with ARNI can further reduce the risks of heart failure hospitalization and death.
SGLT2 Inhibitors
SGLT2 inhibitors, such as dapagliflozin and empagliflozin, are recommended for symptomatic chronic HFrEF patients, regardless of the presence of type 2 diabetes, to reduce hospitalization and mortality rates.
Beta-Blockers
Beta-blockers, including carvedilol, metoprolol, and bisoprolol, are recommended for all patients with LVEF < 40% unless contraindicated. They should be added to RAS inhibitors and diuretics.
MRAs
MRAs, such as eplerenone and spironolactone, have anti-fibrotic and potassium-sparing diuretic effects. They can be used in symptomatic patients without severe renal impairment who are already receiving ACEIs and beta-blockers. Blood potassium levels should be monitored, as MRAs can cause gynecomastia and breast pain in some male patients.
Ivabradine
Ivabradine is a sinus node If channel inhibitor that reduces heart rate without exerting negative inotropic effects. For patients with persistent symptoms despite optimal treatment with beta-blockers, ACEIs/ARBs, and MRAs, or for those who are intolerant or have contraindications to beta-blockers, ivabradine can be added if LVEF ≤ 35% and resting sinus heart rate remains ≥ 70 bpm. This can improve symptoms and prognosis.
Diuretics
Diuretics effectively alleviate symptoms. They should be started at a low dose, with adjustments based on urine output and body weight changes.
Cardiac Glycosides
Cardiac glycosides are primarily used for patients who remain symptomatic or are intolerant to the above treatments.
Vericiguat
Vericiguat is a soluble guanylate cyclase stimulator that increases cGMP production, exerting vasodilatory, anti-fibrotic, and anti-remodeling effects. For patients with persistent symptoms (NYHA Class III-IV) and recent worsening of symptoms despite optimal medical therapy, vericiguat can reduce HF-related hospitalizations and mortality.
In patients with atrial fibrillation (AF), both DCM and AF are risk factors for thromboembolism, requiring long-term anticoagulation with warfarin or novel oral anticoagulants (NOACs).
Cardiac resynchronization therapy (CRT) involves implanting a pacemaker with a left ventricular lead to achieve synchronized contraction of the left and right ventricles. It is highly effective for certain HF patients.
For patients with severe HF unresponsive to medical therapy, heart transplantation can be considered. Ventricular assist devices (VADs) can be used as a bridging therapy to transplantation or as long-term support for end-stage patients who are not candidates for transplantation.
Indications for implantable cardioverter-defibrillators (ICDs) include:
- History of cardiac arrest
- History of sustained ventricular tachycardia (VT), with or without syncope
- LVEF ≤ 35%, NYHA Class II-III, and an expected survival ≥ 1 year
It is important to note that DCM patients carrying high-risk pathogenic mutations (e.g., LMNA, EMD, TMEM43, DSP, RBM20, PLN, FLNC truncating variants) should be considered at high risk for sudden cardiac death (SCD). Even if LVEF > 35%, primary prevention with an ICD should be considered, especially if additional risk factors (e.g., ventricular tachycardia, significant LGE on CMR) are present.
Etiology-specific DCM
Some patients presenting with DCM have identifiable underlying causes. Common examples include:
- Alcoholic cardiomyopathy
- Peripartum cardiomyopathy (PPCM)
- Noncompaction cardiomyopathy (NCCM)
- Takotsubo cardiomyopathy
- Tachycardia-induced cardiomyopathy
Alcoholic Cardiomyopathy
Chronic heavy alcohol consumption can lead to this condition. Diagnostic criteria include:
- Clinical features consistent with DCM
- A history of long-term excessive alcohol intake (WHO criteria: >40 g/day for females, >80 g/day for males; drinking duration > 5 years)
- No other cardiac history or evidence of other causes of DCM (e.g., connective tissue disorders, endocrine diseases)
Early abstinence from alcohol can lead to gradual improvement or recovery in most patients.
Peripartum Cardiomyopathy
This condition occurs in previously healthy females between the last month of pregnancy and up to 5 months postpartum, with clinical features consistent with DCM. It shows a marked racial predisposition, being more common in black females. Risk factors include advanced maternal age, malnutrition, recent pregnancy-induced hypertension, multiple pregnancies, and the use of uterine contraction inhibitors. Recurrence is common with subsequent pregnancies.
Noncompaction Cardiomyopathy
This is caused by the premature arrest of the compaction process from the epicardium to the endocardium during embryonic development. Clinical presentation is similar to DCM. On TTE, the ratio of the non-compacted to compacted myocardial layers is >2, but its accuracy is low. CMR is the most effective diagnostic tool. Management is similar to that of HF.
Takotsubo Cardiomyopathy
This condition, also known as broken heart syndrome, is triggered by emotional stress or psychological stimuli. It presents with sudden chest pain, ECG changes (ST-segment elevation or depression), and absence of significant coronary artery stenosis on angiography. Ventriculography or TTE shows systolic ballooning of the mid and apical segments of the ventricle. Most patients recover normal left ventricular function with psychological support and symptomatic treatment, and the prognosis is generally good.
Tachycardia-induced Cardiomyopathy
This is commonly associated with atrial fibrillation or supraventricular tachycardia and presents with clinical features of DCM. Effective management involves controlling the ventricular rate or performing catheter ablation. Medications to block neurohormonal activation are also necessary.